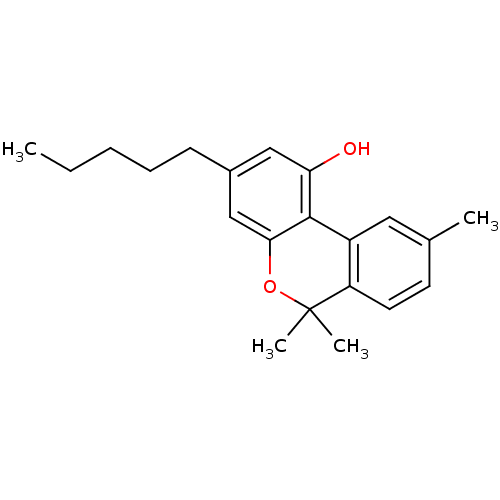
BDBM50061117 6,6,9-Trimethyl-3-pentyl-6H-benzo[c]chromen-1-ol::6,6,9-Trimethyl-3-pentyl-6H-benzo[c]chromen-1-ol (Cannabinol)::6,6,9-Trimethyl-3-pentyl-6H-benzo[c]chromen-1-ol(Cannabinol)::CANNABINOL::CBN::CHEMBL74415
SMILES CCCCCc1cc(O)c-2c(OC(C)(C)c3ccc(C)cc-23)c1
InChI Key InChIKey=VBGLYOIFKLUMQG-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 21 hits for monomerid = 50061117
Found 21 hits for monomerid = 50061117
Affinity DataKi: 70nMAssay Description:Binding affinity to to CB1 (unknown origin) expressed in CHO -k1 cells assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 70nMAssay Description:Binding affinity to to CB2 receptor (unknown origin) expressed in CHO -k1 cells assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 300nMAssay Description:Binding affinity to to CB2 receptor (unknown origin) expressed in AtT20 cells assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Non competitive inhibition of recombinant human CYP2A6 using coumarin as substrate preincubated with NADPH for 20 mins and measured after 30 min by L...More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:Binding affinity to CB1 (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 2.55E+3nMAssay Description:Inhibition of recombinant human CYP2B6 using coumarin as substrate preincubated with NADPH for 20 mins and measured after 30 min by Lineweaver-burk p...More data for this Ligand-Target Pair
Affinity DataKi: 3.90E+4nMAssay Description:Mixed inhibition of recombinant human CYP2B6 using coumarin as substrate preincubated with NADPH for 20 mins and measured after 30 min by Lineweaver-...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Scientus Pharma
Curated by ChEMBL
Scientus Pharma
Curated by ChEMBL
Affinity DataEC50: 6.20E+3nMAssay Description:Agonist activity at ionomycin-stimulated TRPV1 (unknown origin) activationMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Scientus Pharma
Curated by ChEMBL
Scientus Pharma
Curated by ChEMBL
Affinity DataIC50: 8.20E+4nMAssay Description:Agonist activity at ionomycin-stimulated TRPV1 (unknown origin) channel desensitization in presence of capsaicinMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 2(Homo sapiens)
Scientus Pharma
Curated by ChEMBL
Scientus Pharma
Curated by ChEMBL
Affinity DataEC50: 1.90E+4nMAssay Description:Agonist activity at ionomycin-stimulated TRPV2 (unknown origin) activationMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 2(Homo sapiens)
Scientus Pharma
Curated by ChEMBL
Scientus Pharma
Curated by ChEMBL
Affinity DataIC50: 1.57E+4nMAssay Description:Agonist activity at ionomycin-stimulated TRPV2 (unknown origin) channel desensitization in presence of lysophosphatidylcholineMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Scientus Pharma
Curated by ChEMBL
Scientus Pharma
Curated by ChEMBL
Affinity DataEC50: 5.30E+3nMAssay Description:Agonist activity at ionomycin-stimulated TRPV3 (unknown origin) activationMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Scientus Pharma
Curated by ChEMBL
Scientus Pharma
Curated by ChEMBL
Affinity DataIC50: 9.40E+3nMAssay Description:Agonist activity at ionomycin-stimulated TRPV3 (unknown origin) channel desensitization in presence of carvacrolMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Scientus Pharma
Curated by ChEMBL
Scientus Pharma
Curated by ChEMBL
Affinity DataEC50: 1.61E+4nMAssay Description:Agonist activity at ionomycin-stimulated TRPV4 (unknown origin) activationMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Scientus Pharma
Curated by ChEMBL
Scientus Pharma
Curated by ChEMBL
Affinity DataIC50: 5.40E+3nMAssay Description:Agonist activity at ionomycin-stimulated TRPV4 (unknown origin) channel desensitization in presence of 4alphaPDDMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Scientus Pharma
Curated by ChEMBL
Scientus Pharma
Curated by ChEMBL
Affinity DataEC50: 180nMAssay Description:Agonist activity at TRPA1 (unknown origin) activationMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Scientus Pharma
Curated by ChEMBL
Scientus Pharma
Curated by ChEMBL
Affinity DataIC50: 400nMAssay Description:Agonist activity at TRPA1 (unknown origin) channel desensitization in presence of isothiocyanateMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Scientus Pharma
Curated by ChEMBL
Scientus Pharma
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Agonist activity at TRPA1 (unknown origin) channel desensitization in presence of icilinMore data for this Ligand-Target Pair
Affinity DataEC50: 60nMAssay Description:Displacement of 35S]GTPgammaS from human CB2 receptor transfected in CHO cells assessed as inhibition forskolin-stimulated cyclic AMP productionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMAssay Description:Non competitive inhibition of CYP2J2 (unknown origin) in presence of NADPH by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.04E+4nMAssay Description:Non competitive inhibition of recombinant human CYP2A6 using coumarin as substrate preincubated with NADPH for 20 mins and measured after 30 minMore data for this Ligand-Target Pair