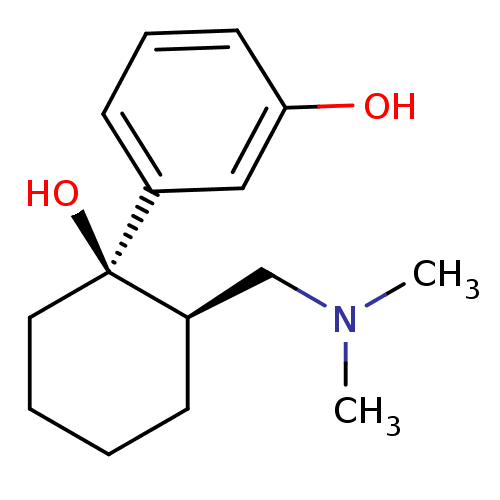
BDBM50176263 3-((1R,2R)-2-((dimethylamino)methyl)-1-hydroxycyclohexyl)phenol::CHEMBL1400::O-DESMETHYL TRAMADOL::Ultracet::rel-3-((1R,2R)-2-((dimethylamino)methyl)-1-hydroxycyclohexyl)phenol
SMILES CN(C)C[C@H]1CCCC[C@]1(O)c1cccc(O)c1
InChI Key InChIKey=UWJUQVWARXYRCG-HIFRSBDPSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50176263
Found 9 hits for monomerid = 50176263
TargetMu-type opioid receptor(Homo sapiens (Human))
Rensselaer Polytechnic Institute
Curated by ChEMBL
Rensselaer Polytechnic Institute
Curated by ChEMBL
Affinity DataKi: 8.60nMAssay Description:Activity at human cloned mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
Rensselaer Polytechnic Institute
Curated by ChEMBL
Rensselaer Polytechnic Institute
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Displacement of [3H]naloxone from human MOR expressed in CHO-K1 cell membranes after 60 mins by microbeta scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Displacement of [3H]-DAMGO from rat mu opioid receptor expressed in CHO cells incubated for 30 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
Rensselaer Polytechnic Institute
Curated by ChEMBL
Rensselaer Polytechnic Institute
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 60 minsMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
Rensselaer Polytechnic Institute
Curated by ChEMBL
Rensselaer Polytechnic Institute
Curated by ChEMBL
Affinity DataKi: 450nMAssay Description:Activity at human cloned kappa opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
Rensselaer Polytechnic Institute
Curated by ChEMBL
Rensselaer Polytechnic Institute
Curated by ChEMBL
Affinity DataKi: 2.90E+3nMAssay Description:Displacement of [3H]Naltrindole form human delta opioid receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
Rensselaer Polytechnic Institute
Curated by ChEMBL
Rensselaer Polytechnic Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]U69593 from human KOR expressed in CHO cell membranes after 60 minsMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
Rensselaer Polytechnic Institute
Curated by ChEMBL
Rensselaer Polytechnic Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]DPDPE from human DOR expressed in CHO cell membranes after 60 minsMore data for this Ligand-Target Pair