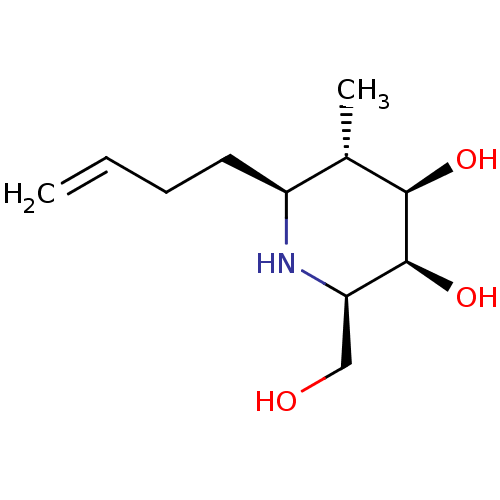
BDBM50320827 CHEMBL1163562::beta-1-C-butenyl-1-deoxygalactonojirimycin
SMILES C[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)N[C@H]1CCC=C
InChI Key InChIKey=DJRPURFURDDUBK-DAWVFNFOSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50320827
Found 4 hits for monomerid = 50320827
TargetProbable alpha-glucosidase Os06g0675700(Oryza sativa subsp. japonica)
University Of Toyama
Curated by ChEMBL
University Of Toyama
Curated by ChEMBL
Affinity DataIC50: 4.50E+4nMpH: 5.0Assay Description:Inhibition of rice alpha-glucosidase assessed as D-glucose release at pH 5 after 10 to 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+4nMAssay Description:Inhibition of human lysosome alpha-galactosidase assessed as p-nitrophenol release by spectrometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMpH: 6.5Assay Description:Inhibition of coffee bean alpha-galactosidase assessed as p-nitrophenol release at pH 6.5 by spectrometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMpH: 5.0Assay Description:Inhibition of Caldocellum saccharolyticum beta-glucosidase assessed as p-nitrophenol release at pH 5 by spectrometric analysisMore data for this Ligand-Target Pair