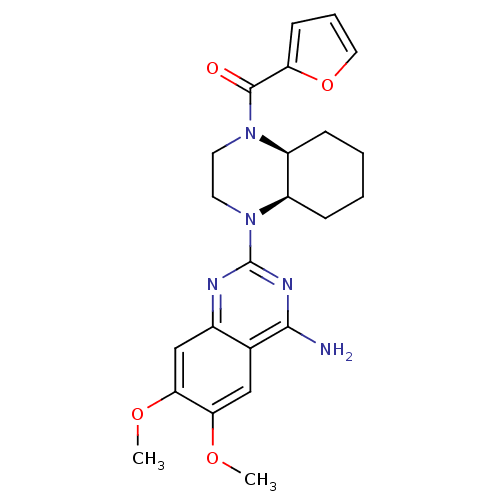
BDBM50403649 CYCLAZOSIN
SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN([C@H]2CCCC[C@@H]12)C(=O)c1ccco1
InChI Key InChIKey=XBRXTUGRUXGBPX-DLBZAZTESA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50403649
Found 3 hits for monomerid = 50403649
TargetAlpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor(RAT)
University Of Bologna
Curated by ChEMBL
University Of Bologna
Curated by ChEMBL
Affinity DataKi: 741nMAssay Description:Antagonistic affinity against native rat cortex Alpha-2 adrenergic receptorMore data for this Ligand-Target Pair
Affinity DataKi: <1.00E+3nMAssay Description:Antagonistic affinity against cloned human 5-hydroxytryptamine 1A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 8.32E+3nMAssay Description:Antagonistic affinity against rat striatum Dopamine receptor D2More data for this Ligand-Target Pair