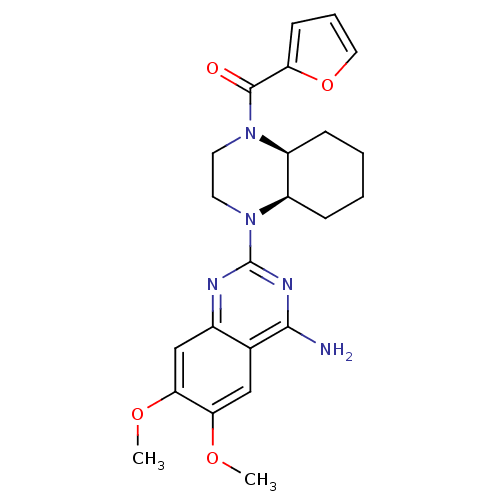
BDBM50403649 CYCLAZOSIN
SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN([C@H]2CCCC[C@@H]12)C(=O)c1ccco1
InChI Key InChIKey=XBRXTUGRUXGBPX-DLBZAZTESA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50403649
Found 3 hits for monomerid = 50403649
Affinity DataKi: 0.170nMAssay Description:Displacement of [3H]prazosin from human Alpha-1D adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.324nMAssay Description:Displacement of [3H]prazosin from human Alpha-1B adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5.5nMAssay Description:Displacement of [3H]prazosin from human alpha1A adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair