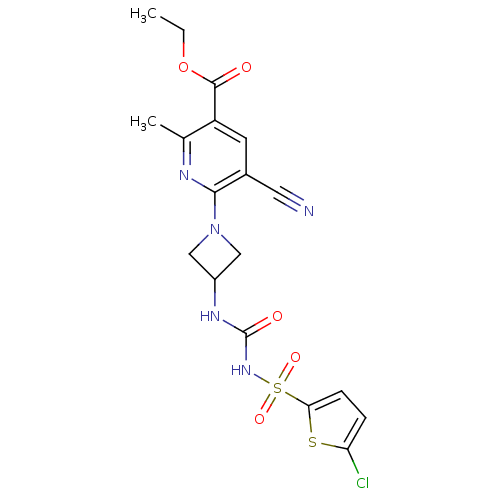
BDBM50436963 CHEMBL2402255
SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CC(C1)NC(=O)NS(=O)(=O)c1ccc(Cl)s1
InChI Key InChIKey=DXRGSOKOVRDWNW-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50436963
Found 4 hits for monomerid = 50436963
Affinity DataIC50: 25nMAssay Description:Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human P2Y12More data for this Ligand-Target Pair
Affinity DataIC50: 9.5nMAssay Description:Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of fibrinogen-induced aggregationMore data for this Ligand-Target Pair
Affinity DataIC50: 6.30nMAssay Description:Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair