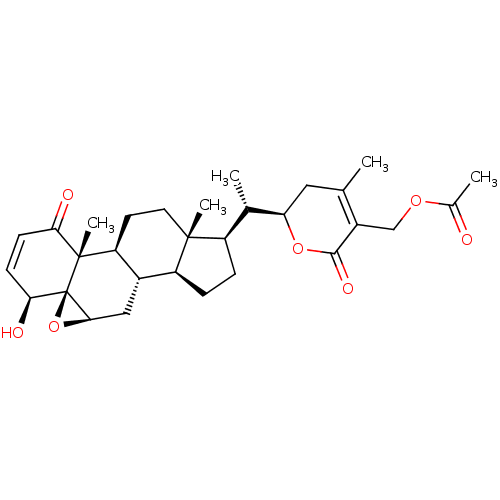
BDBM50514011 CHEMBL2047407
SMILES [H][C@@]12C[C@@]3([H])[C@]4([H])CC[C@]([H])([C@H](C)[C@@]5([H])CC(C)=C(COC(C)=O)C(=O)O5)[C@@]4(C)CC[C@]3([H])[C@@]3(C)C(=O)C=C[C@H](O)[C@@]13O2
InChI Key InChIKey=ARTYOOFBEGPUAU-NSXHFEBNSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50514011
Found 2 hits for monomerid = 50514011
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of p97 (unknown origin) expressed in Escherichia coli BL21 (DE3) pre-incubated for 10 mins before ATP addition and measured up to 120 mins...More data for this Ligand-Target Pair
TargetATP-dependent Clp protease ATP-binding subunit clpX-like, mitochondrial(Homo sapiens)
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 9.50E+3nMAssay Description:Inhibition of ClpX (unknown origin) expressed in Escherichia coli BL21 (DE3) pre-incubated for 10 mins before ATP addition and measured up to 120 min...More data for this Ligand-Target Pair