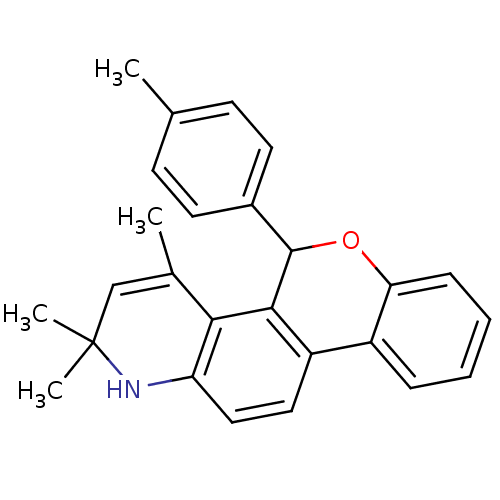
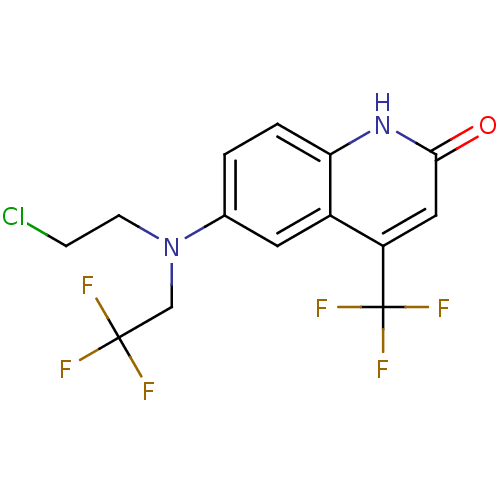
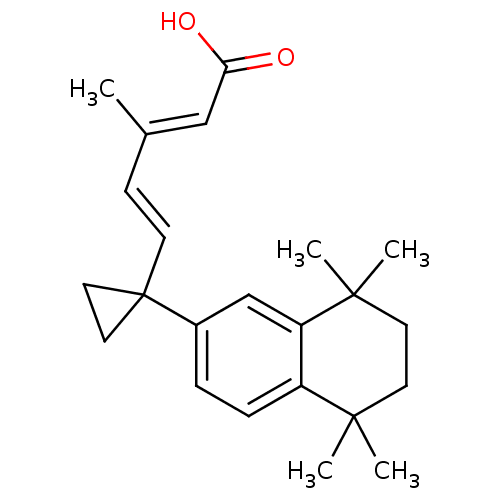
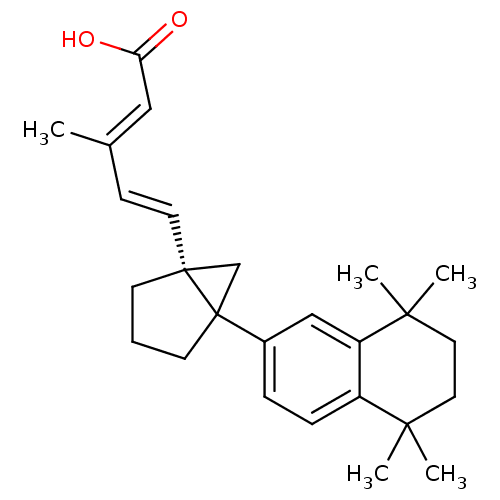
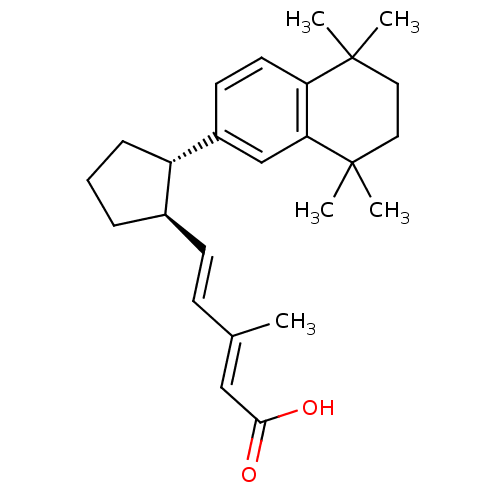
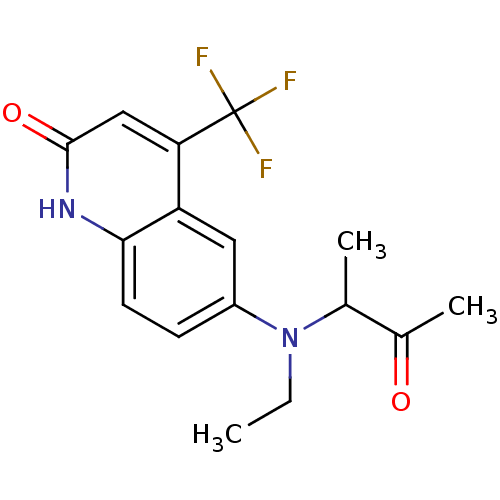
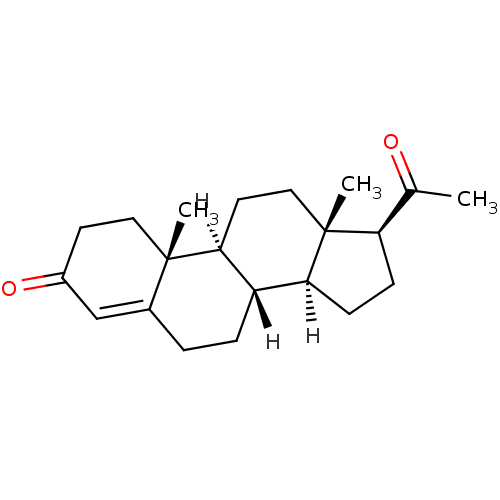
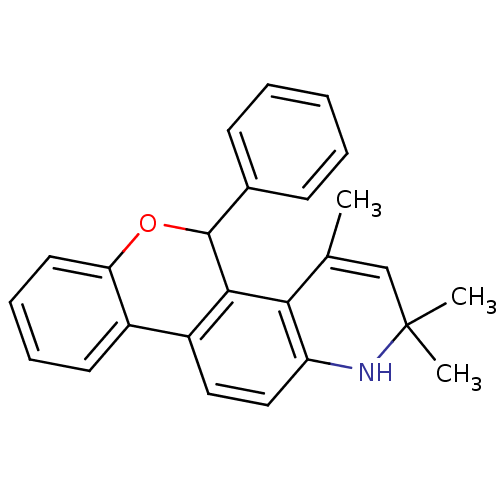
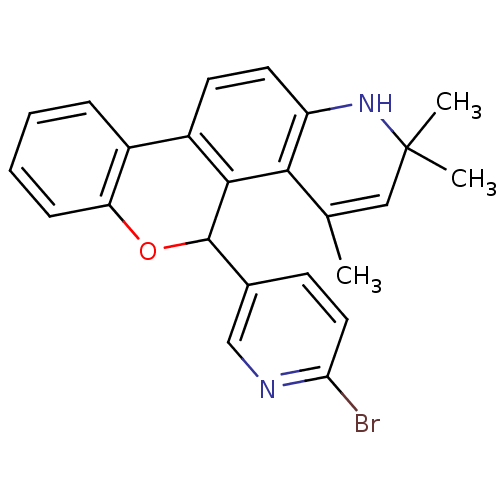
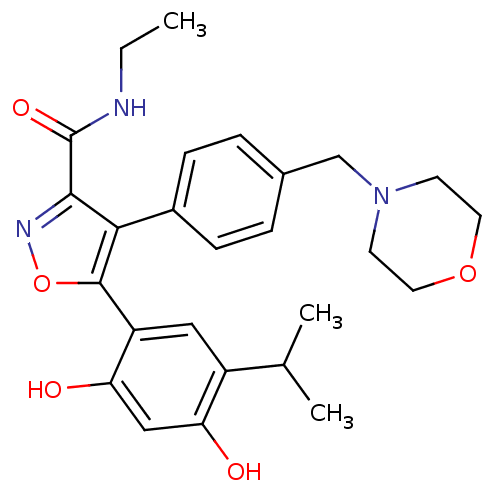
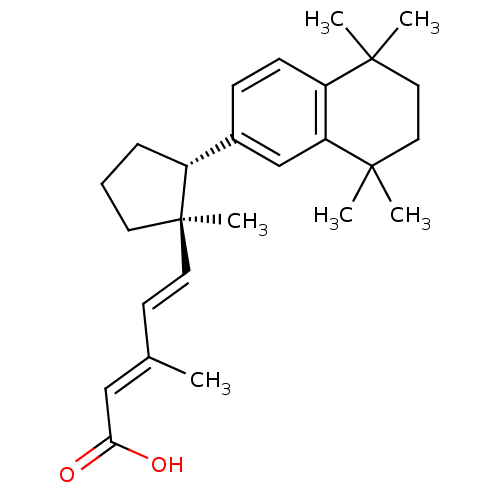
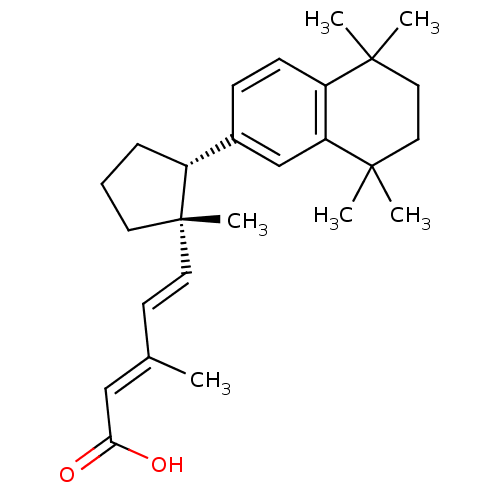
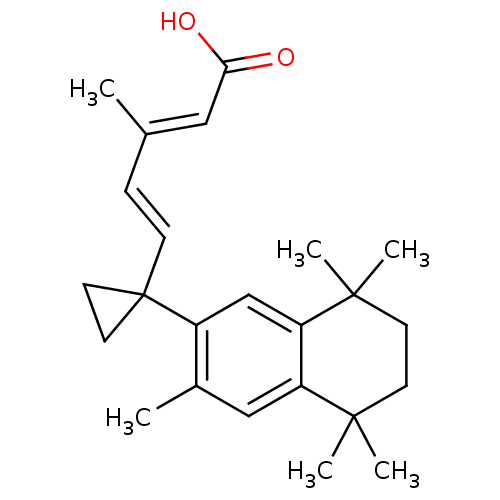
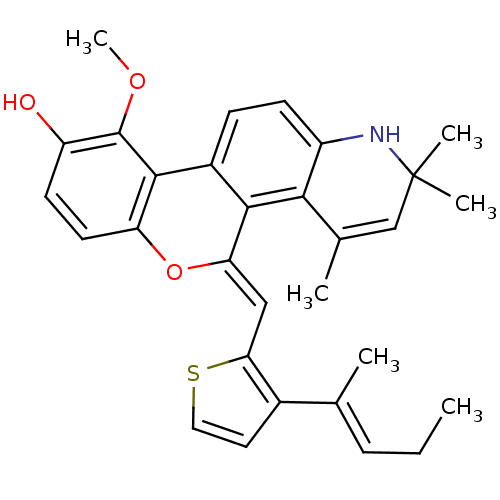
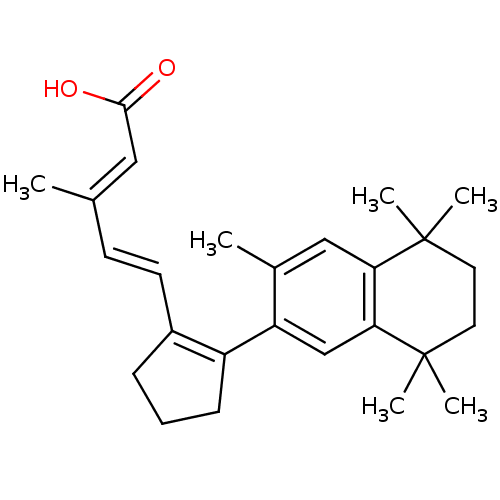
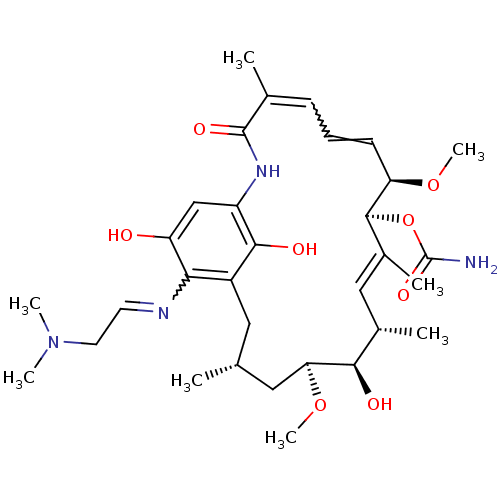
Affinity DataKi: 0.200nM ΔG°: -51.5kJ/mole IC50: 0.200nM EC50: 0.200nMpH: 7.5 T: 2°CAssay Description:Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the...More data for this Ligand-Target Pair
Affinity DataKi: 0.340nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 0.400nM EC50: 0.5nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 0.410nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 0.430nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 0.5nM EC50: 1nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nM EC50: 0.890nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nM ΔG°: -55.2kJ/mole EC50: 0.400nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 0.550nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 0.600nM ΔG°: -48.9kJ/mole IC50: 1.10nM EC50: 5nMpH: 7.5 T: 2°CAssay Description:Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the...More data for this Ligand-Target Pair
Affinity DataKi: 0.690nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 0.700nM ΔG°: -48.6kJ/mole IC50: 0.200nM EC50: 0.200nMpH: 7.5 T: 2°CAssay Description:Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 0.870nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 1.10nM ΔG°: -47.5kJ/mole IC50: 0.200nM EC50: 0.100nMpH: 7.5 T: 2°CAssay Description:Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 1.20nM ΔG°: -47.3kJ/mole IC50: 1.60nM EC50: 1.30nMpH: 7.5 T: 2°CAssay Description:Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 1.40nM ΔG°: -47.0kJ/mole IC50: 0.400nM EC50: 0.300nMpH: 7.5 T: 2°CAssay Description:Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the...More data for this Ligand-Target Pair
Affinity DataKi: 1.40nM EC50: 0.800nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 1.70nM ΔG°: -52.1kJ/mole EC50: 0.200nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 2.10nM ΔG°: -46.0kJ/mole IC50: 1.40nM EC50: 0.200nMpH: 7.5 T: 2°CAssay Description:Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the...More data for this Ligand-Target Pair
Affinity DataKi: 2.30nM ΔG°: -51.3kJ/mole EC50: 0.400nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 2.40nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 2.60nM ΔG°: -51.0kJ/mole EC50: 0.5nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 2.90nM ΔG°: -50.7kJ/mole EC50: 0.900nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 2.90nMAssay Description:Binding affinity was determined on Human androgen receptor (hAR) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Transcriptional activation in CV-1 cells expressing retinoic acid receptor RAR gammaMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alphaMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alphaMore data for this Ligand-Target Pair
Affinity DataKi: 3.10nM EC50: 5.80nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 3.40nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 3.5nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 3.60nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Binding affinity to human N-terminal polyHis-tagged GRP94 (L69 to N337) expressed in Escherichia coli BL21(DE3) after 3 hrs by fluorescence polarizat...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alphaMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alphaMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibition of [3H]-9-cis-RA binding to RXR beta receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibition of [3H]-9-cis-RA binding to RXR alpha receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4.40nM ΔG°: -44.3kJ/mole IC50: 6.10nM EC50: 0.5nMpH: 7.5 T: 2°CAssay Description:Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Effective potency in transcriptional activation assay in CV-1 cells expressing retinoic acid receptor RAR gammaMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alphaMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Binding affinity to human N-terminal polyHis-tagged GRP94 (L69 to N337) expressed in Escherichia coli BL21(DE3) after 3 hrs by fluorescence polarizat...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR gammaMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR alphaMore data for this Ligand-Target Pair

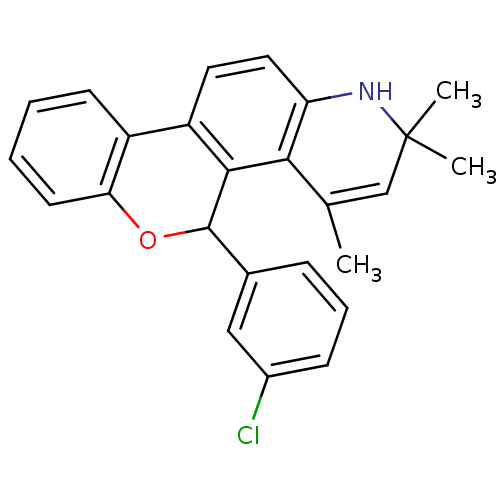
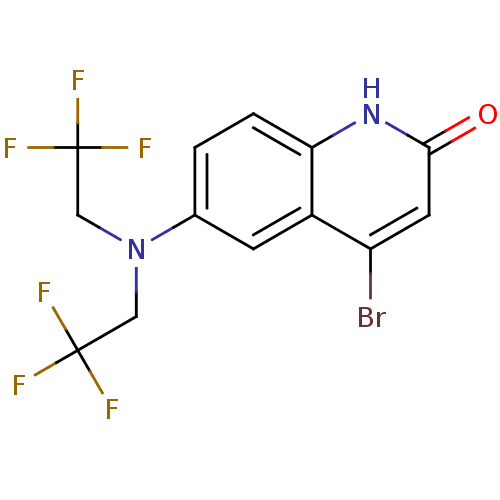
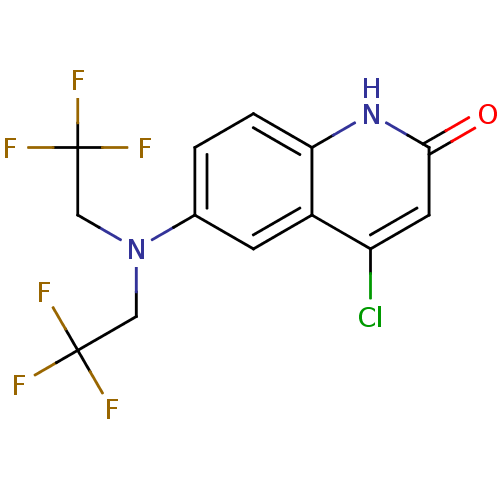
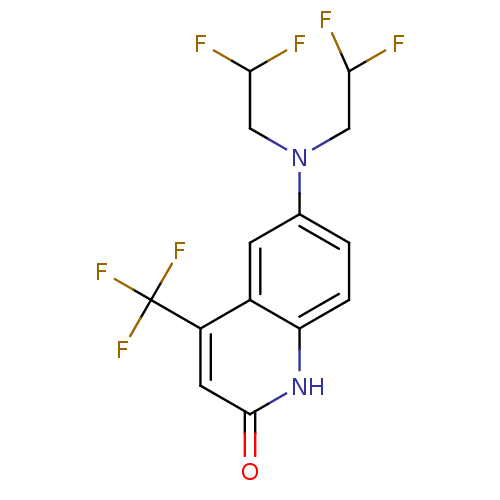
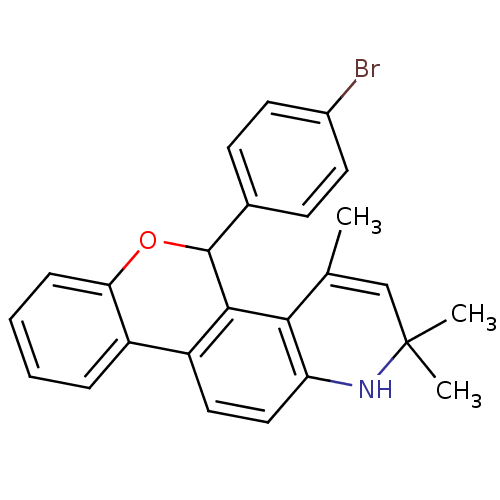
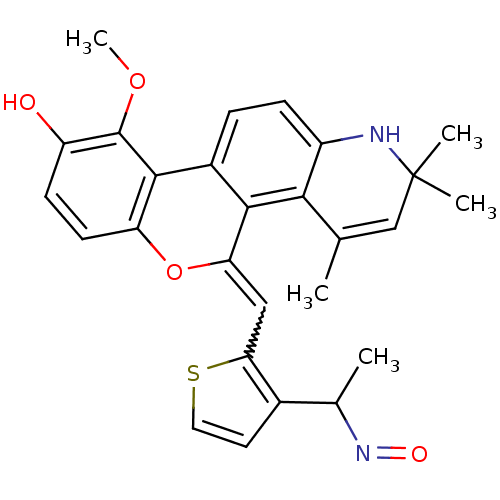
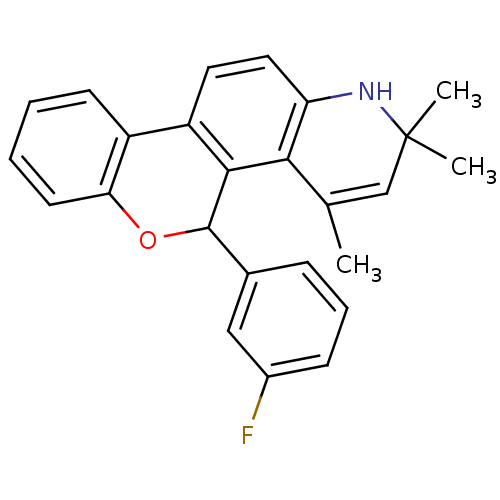
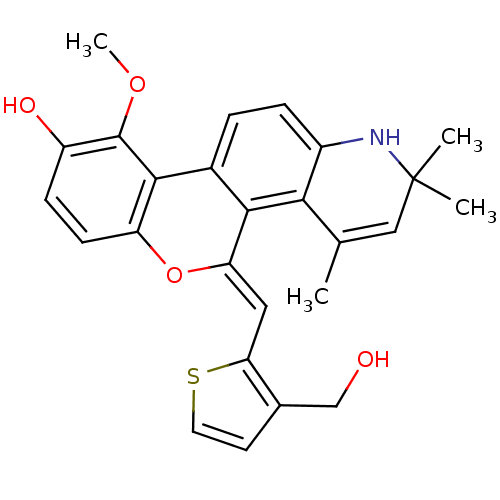
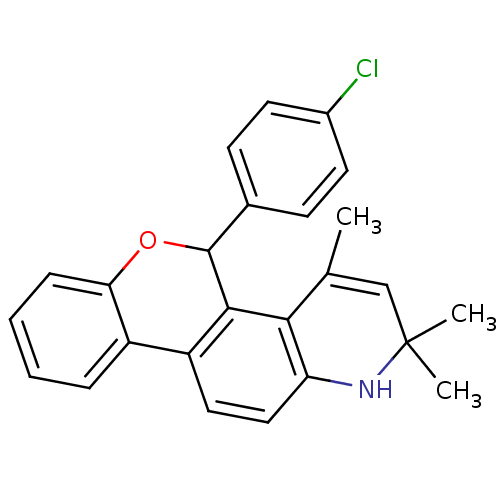
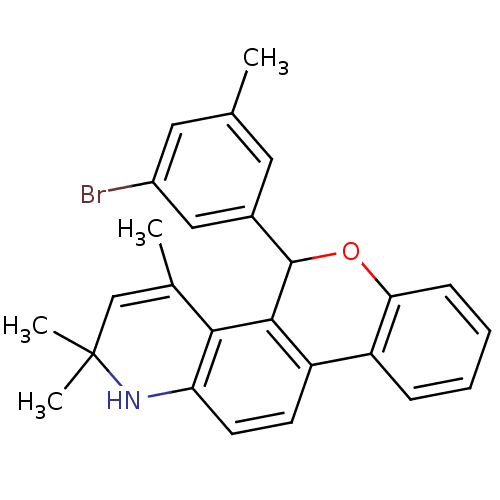
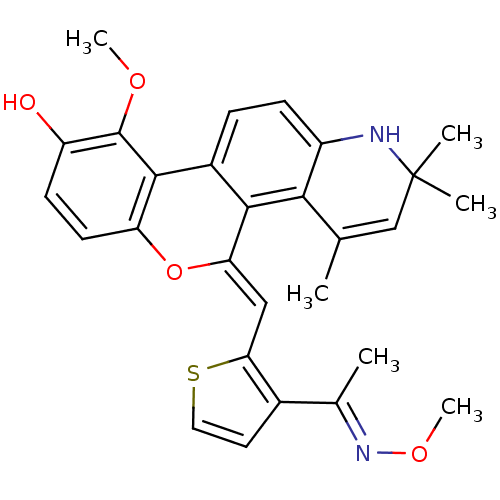


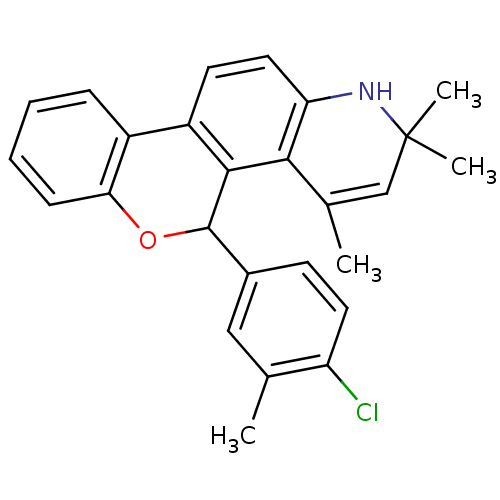
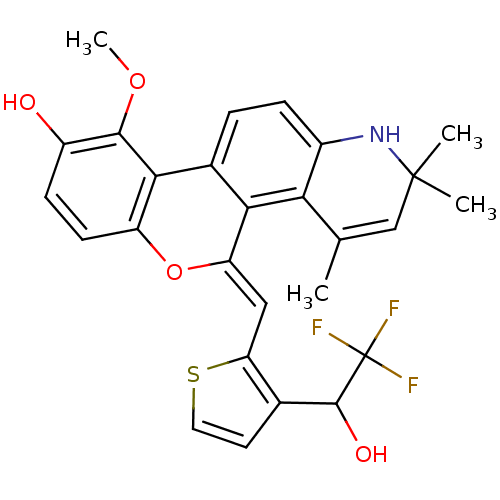

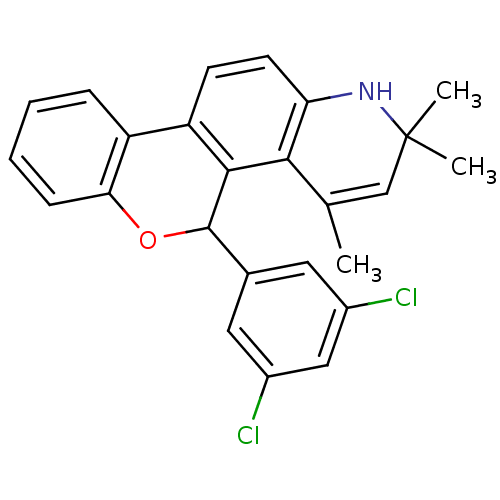
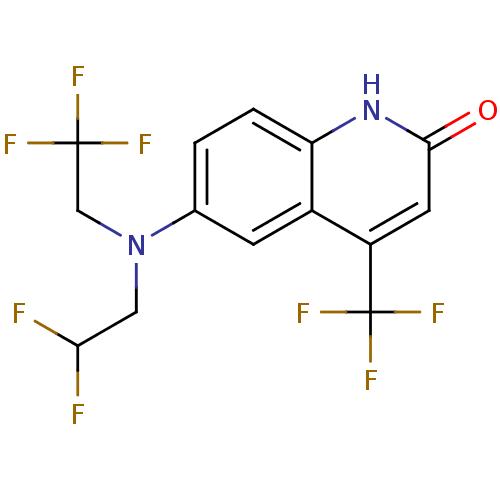

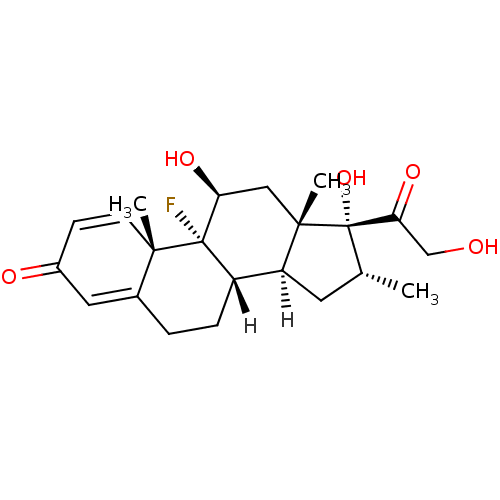
 3D Structure (crystal)
3D Structure (crystal)