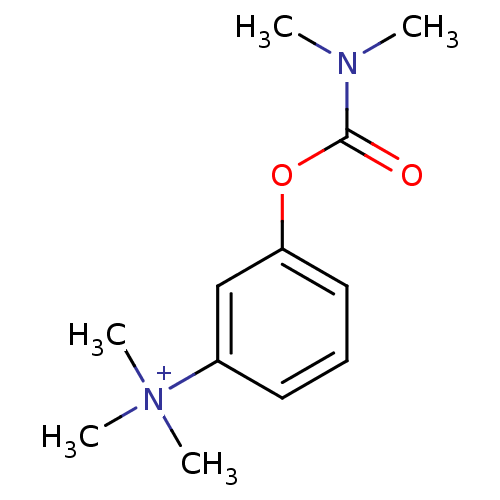
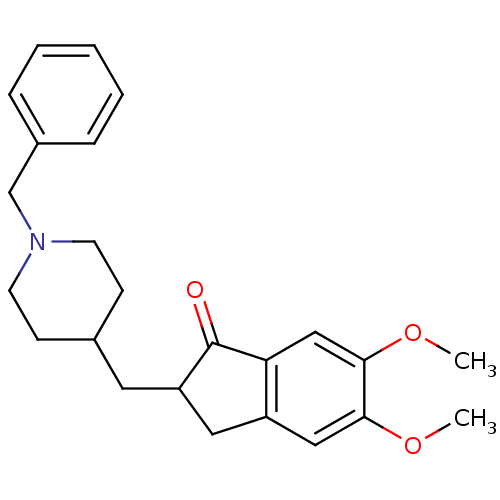
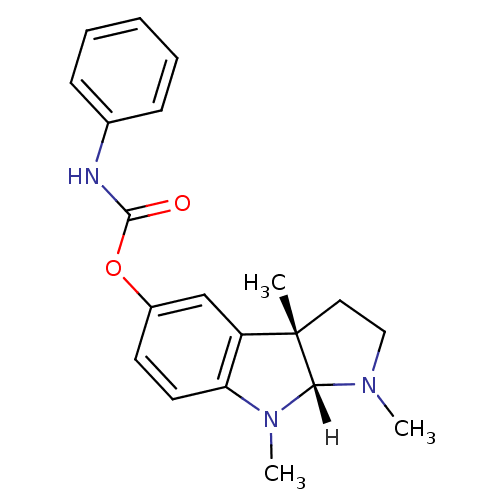
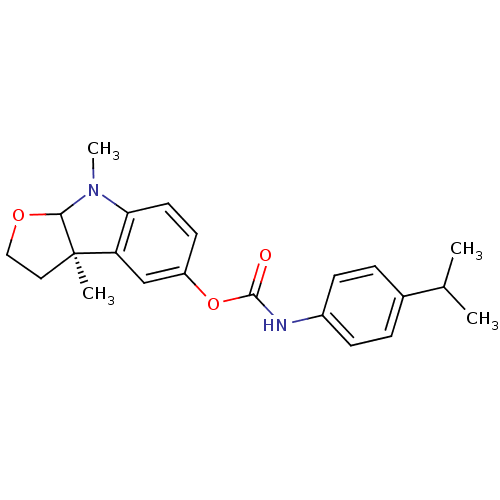
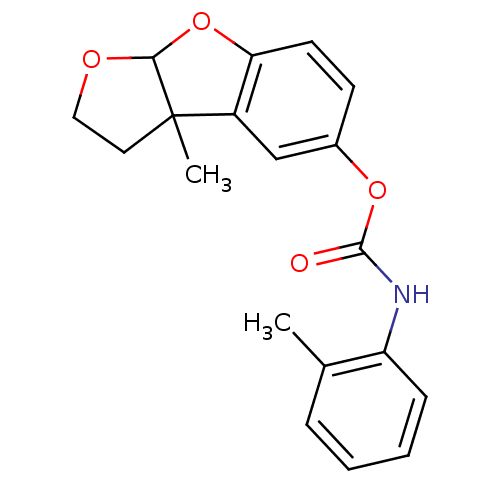
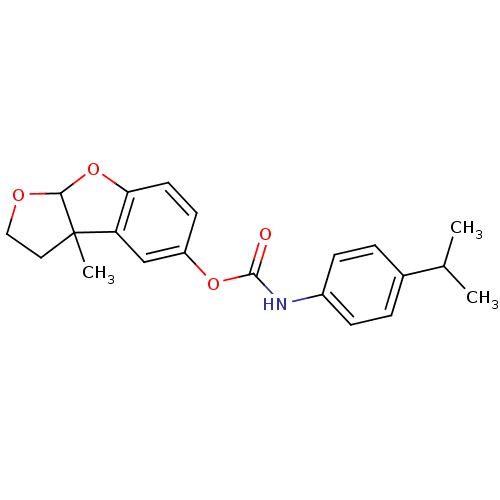
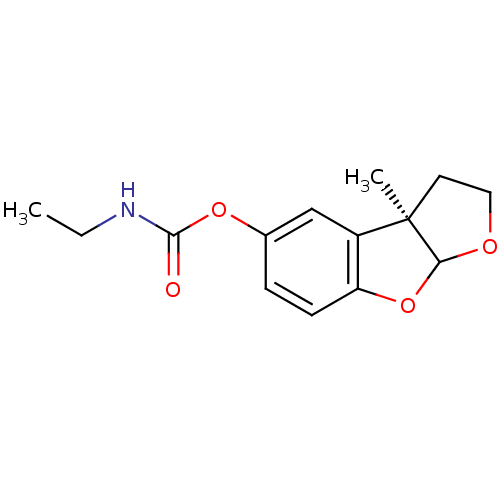
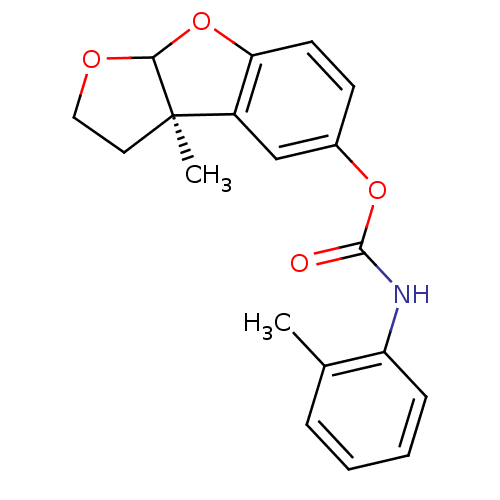
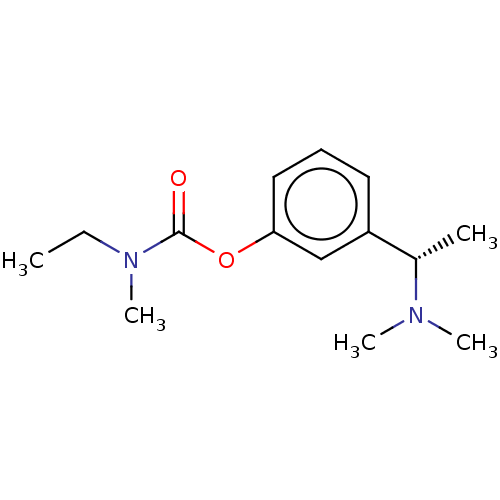
Affinity DataIC50: 2nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibitory concentration against human plasma ButyrylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibitory concentration against human plasma ButyrylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibitory concentration against human plasma ButyrylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibitory concentration against human plasma ButyrylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibitory concentration against human plasma ButyrylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human plasma BChE pretreated for 30 mins by Ellman techniqueMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibitory concentration against human erythrocyte AcetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 10.3nMAssay Description:Inhibition of human whole RBC AChE pretreated for 30 mins by Ellman techniqueMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibitory concentration against human erythrocyte AcetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 14.6nMAssay Description:Inhibition of human whole RBC AChE pretreated for 30 mins by Ellman techniqueMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibitory concentration against human plasma ButyrylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human plasma BChE pretreated for 30 mins by Ellman techniqueMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibitory concentration against human plasma ButyrylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 18.8nMAssay Description:Inhibition of human whole RBC AChE pretreated for 30 mins by Ellman techniqueMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibitory concentration against human plasma ButyrylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibitory concentration against human erythrocyte AcetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibitory concentration against human erythrocyte AcetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibitory concentration against human erythrocyte AcetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Inhibitory concentration against human erythrocyte AcetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibitory concentration against human plasma ButyrylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of human whole RBC AChE pretreated for 30 mins by Ellman techniqueMore data for this Ligand-Target Pair
Affinity DataIC50: 25.4nMAssay Description:Inhibition of human whole RBC AChE pretreated for 30 mins by Ellman techniqueMore data for this Ligand-Target Pair
Affinity DataIC50: 26.1nMAssay Description:Inhibition of human whole RBC AChE pretreated for 30 mins by Ellman techniqueMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibitory concentration against human plasma ButyrylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibitory concentration against human erythrocyte AcetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 27.9nMAssay Description:Inhibition of human whole RBC AChE pretreated for 30 mins by Ellman techniqueMore data for this Ligand-Target Pair
Affinity DataIC50: 28nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Inhibitory concentration against human erythrocyte AcetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 36nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair

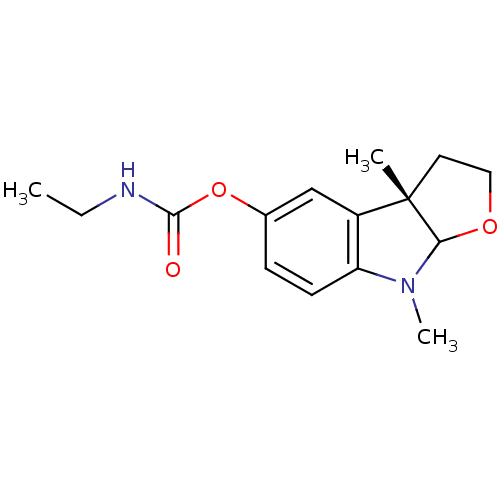

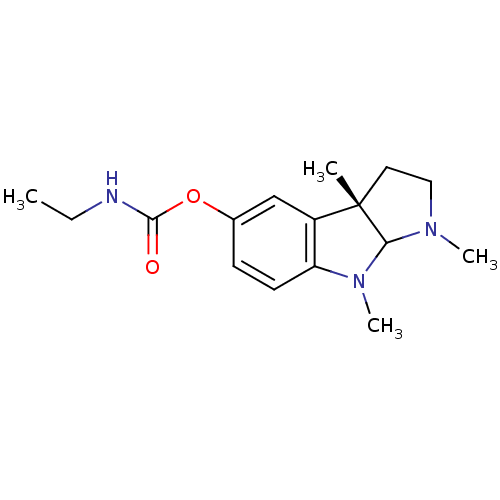
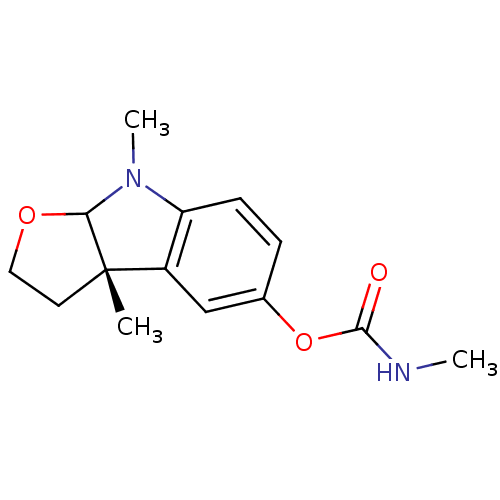
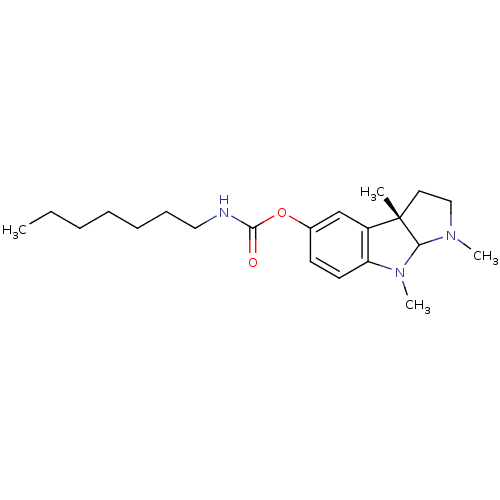
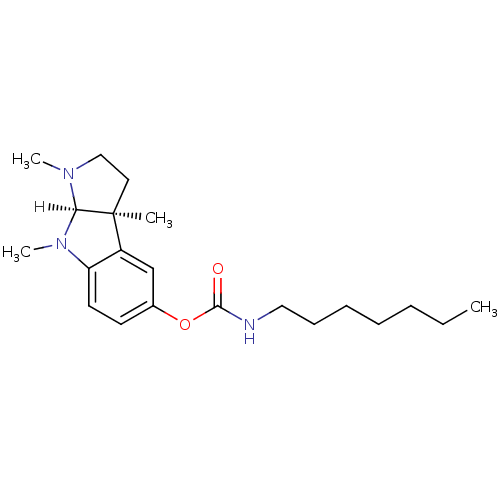
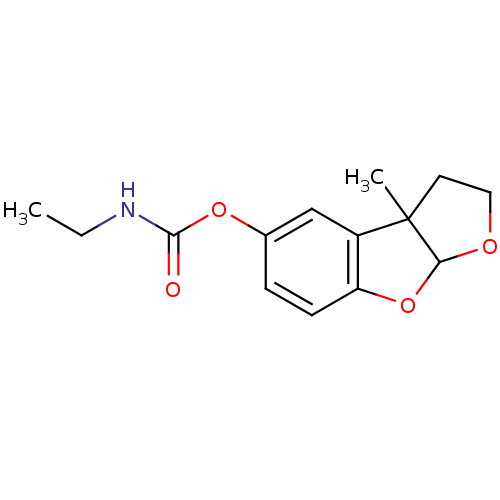
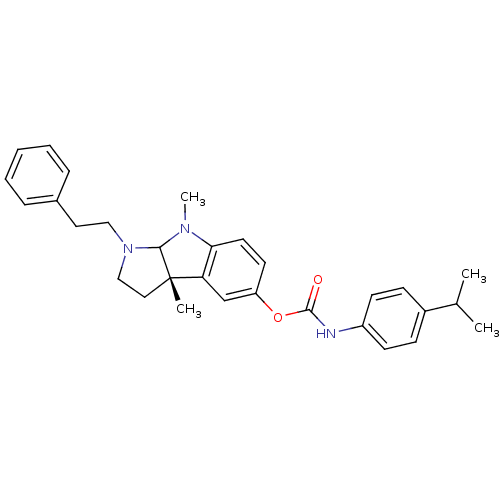
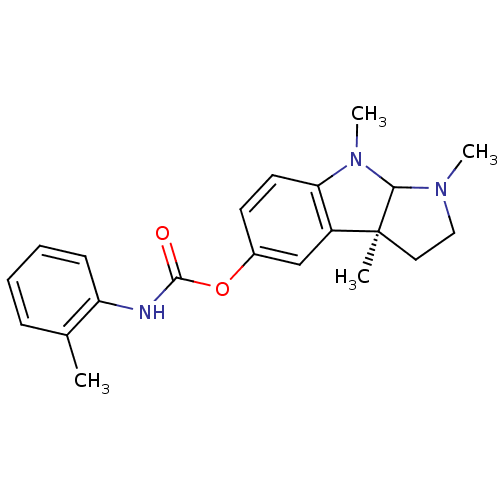


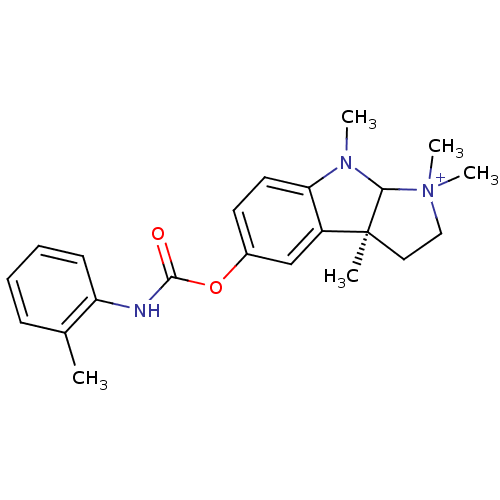
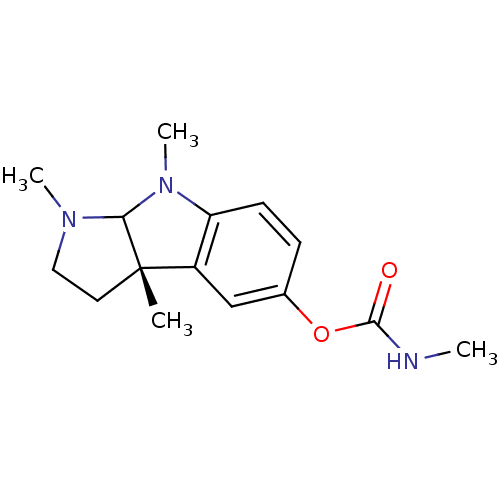
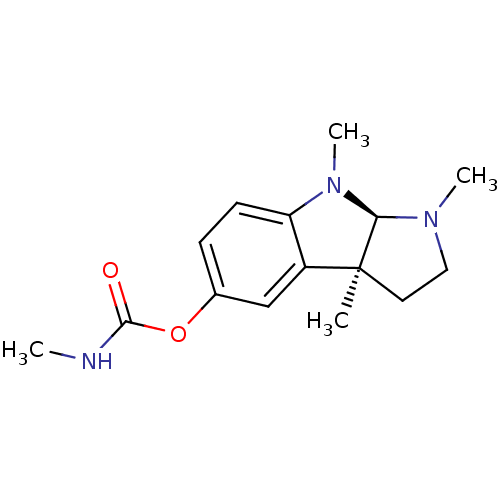
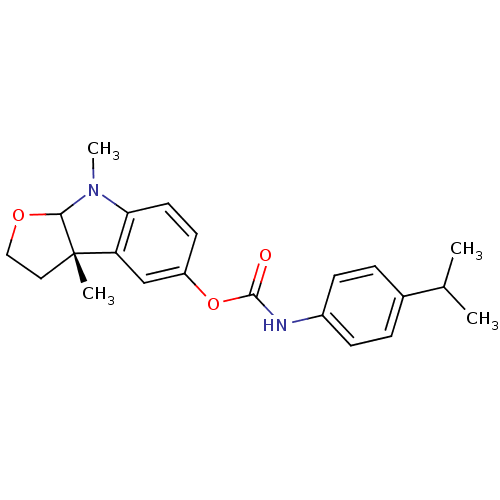
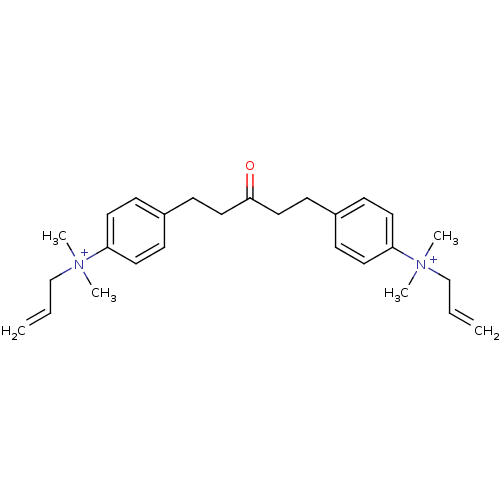
 3D Structure (crystal)
3D Structure (crystal)