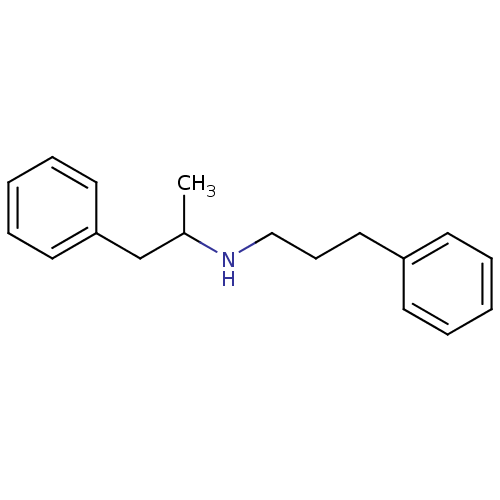
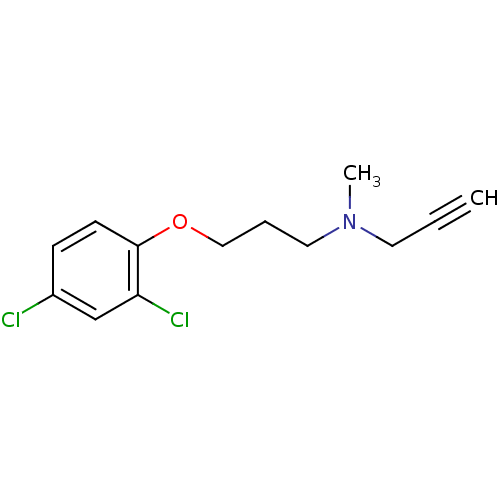
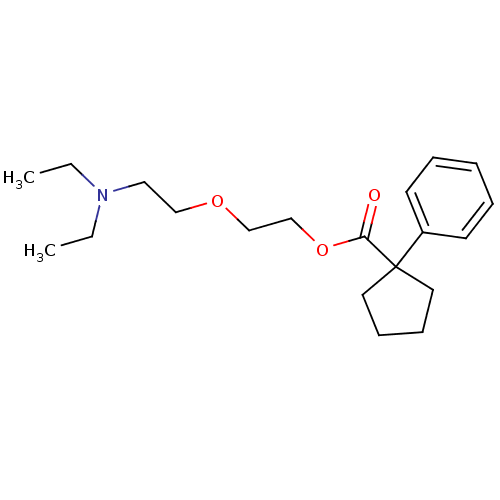
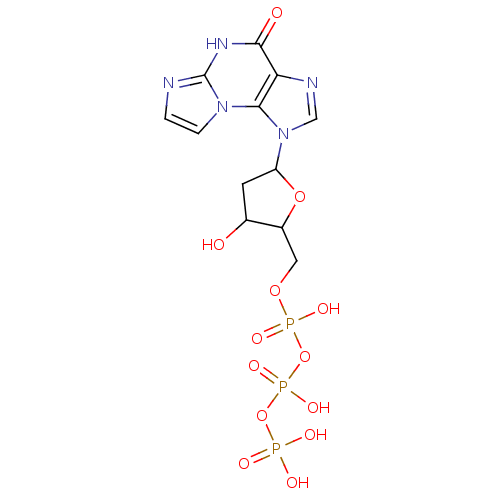
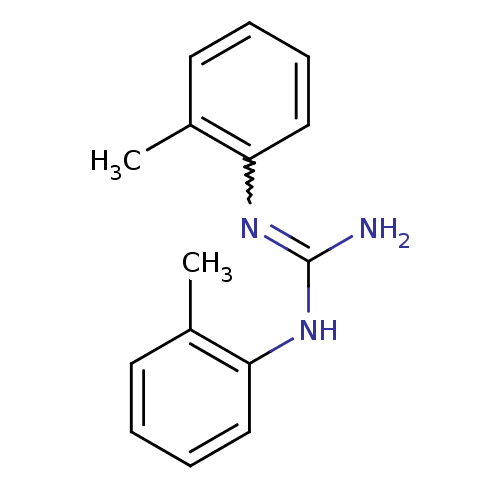
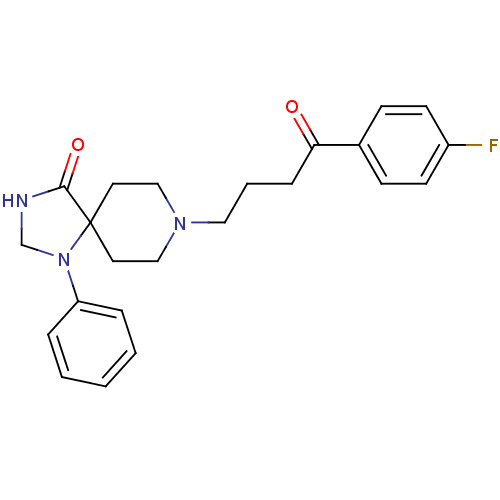
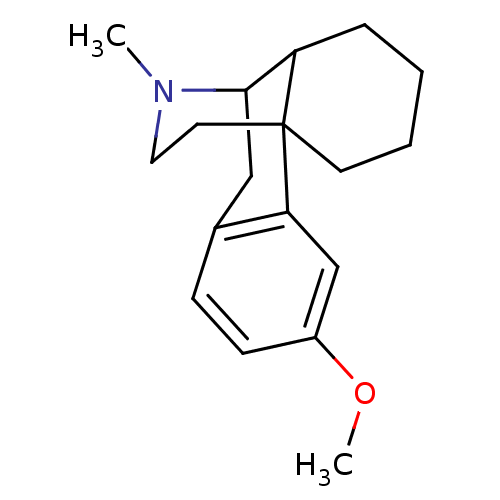
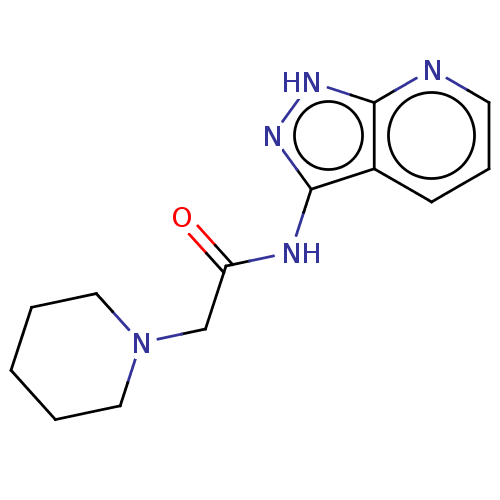
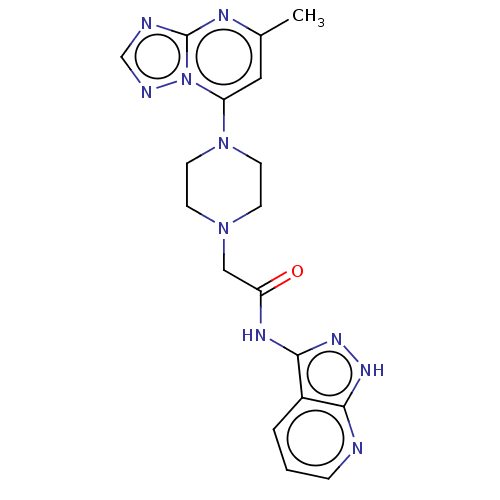
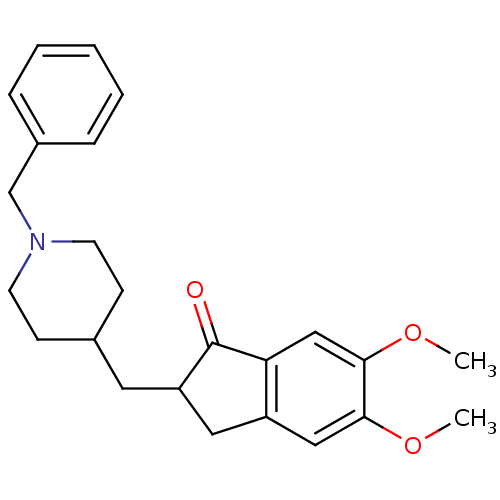
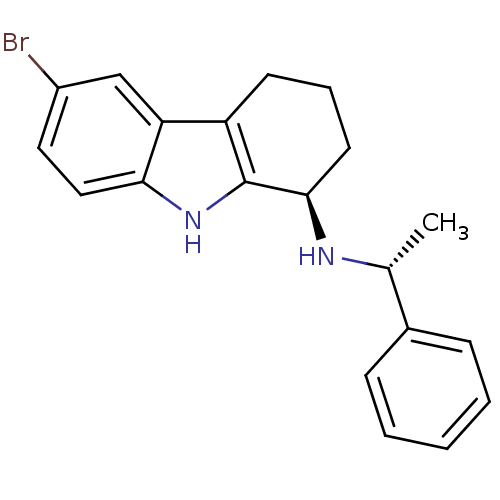
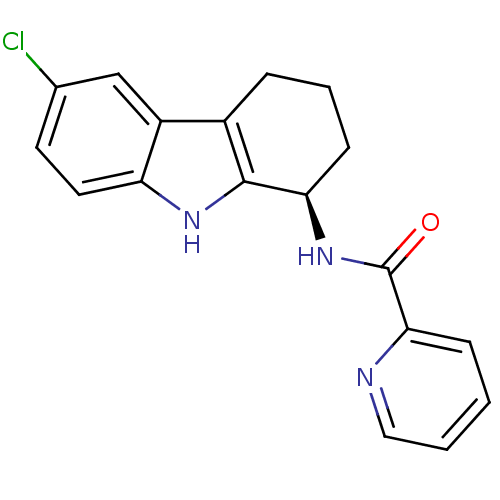
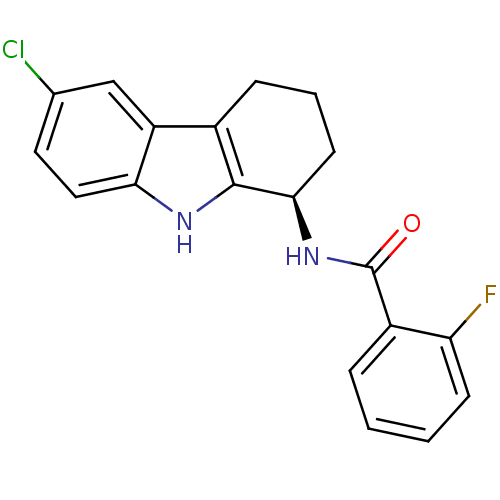
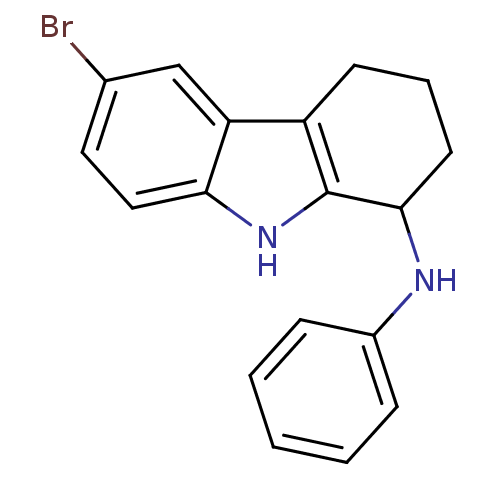
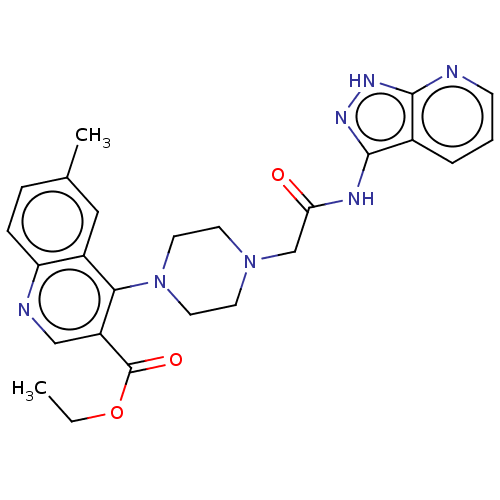
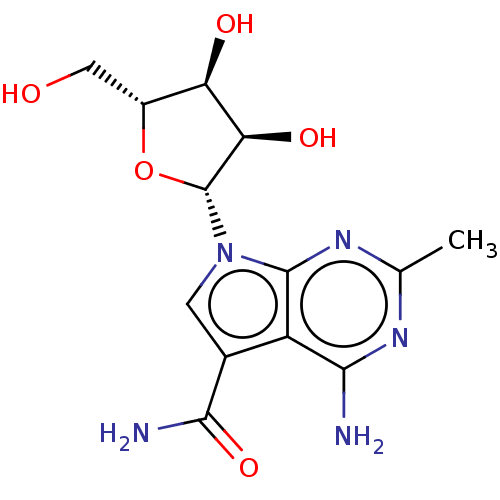
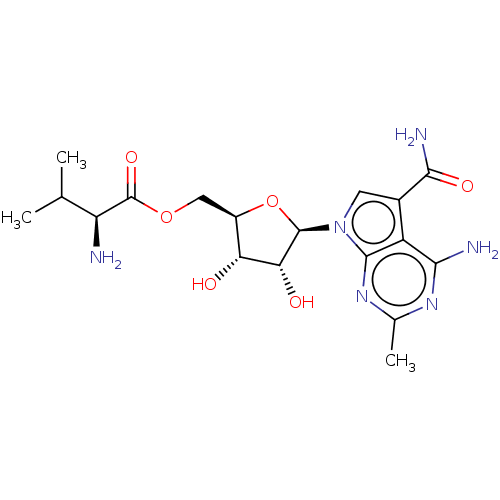
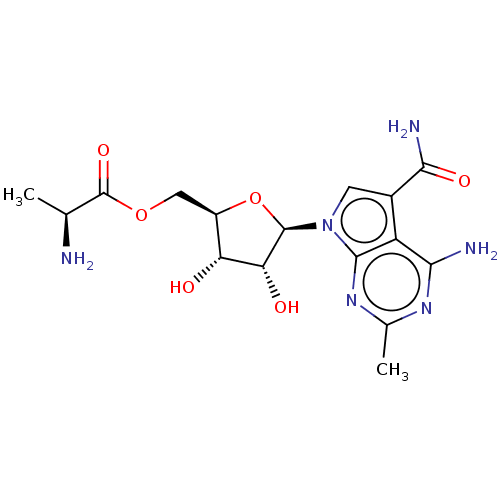
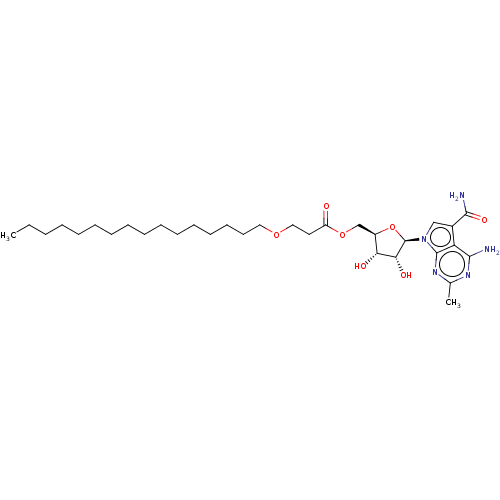
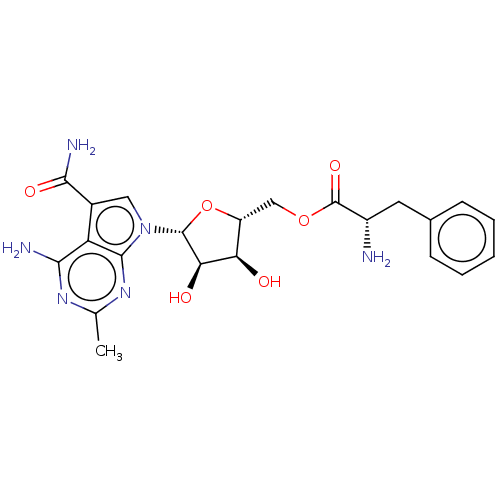
TargetSigma non-opioid intracellular receptor 1(Homo sapiens (Human))
Medical College of Georgia
Curated by PDSP Ki Database
Medical College of Georgia
Curated by PDSP Ki Database
TargetSigma non-opioid intracellular receptor 1(Homo sapiens (Human))
Medical College of Georgia
Curated by PDSP Ki Database
Medical College of Georgia
Curated by PDSP Ki Database
TargetSigma non-opioid intracellular receptor 1(Homo sapiens (Human))
Medical College of Georgia
Curated by PDSP Ki Database
Medical College of Georgia
Curated by PDSP Ki Database
TargetSigma non-opioid intracellular receptor 1(Homo sapiens (Human))
Medical College of Georgia
Curated by PDSP Ki Database
Medical College of Georgia
Curated by PDSP Ki Database
TargetSigma non-opioid intracellular receptor 1(Homo sapiens (Human))
Medical College of Georgia
Curated by PDSP Ki Database
Medical College of Georgia
Curated by PDSP Ki Database
TargetSigma non-opioid intracellular receptor 1(Homo sapiens (Human))
Medical College of Georgia
Curated by PDSP Ki Database
Medical College of Georgia
Curated by PDSP Ki Database
TargetSigma non-opioid intracellular receptor 1(Homo sapiens (Human))
Medical College of Georgia
Curated by PDSP Ki Database
Medical College of Georgia
Curated by PDSP Ki Database
TargetSigma non-opioid intracellular receptor 1(Homo sapiens (Human))
Medical College of Georgia
Curated by PDSP Ki Database
Medical College of Georgia
Curated by PDSP Ki Database
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: 4.80nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: 4.90nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: 45nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: 49nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: 113nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: 8.71E+3nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAmyloid-beta precursor protein(Homo sapiens (Human))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: 1.57E+4nMAssay Description:Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation incubated for 48 hrs by thioflavin-T fluorescence assayMore data for this Ligand-Target Pair
TargetAmyloid-beta precursor protein(Homo sapiens (Human))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: 1.79E+4nMAssay Description:Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation incubated for 48 hrs by thioflavin-T fluorescence assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Jamia Millia Islamia (Central University)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 m...More data for this Ligand-Target Pair
Affinity DataEC50: 2.65E+3nMAssay Description:Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05...More data for this Ligand-Target Pair
Affinity DataEC50: 3.03E+3nMAssay Description:Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05...More data for this Ligand-Target Pair
Affinity DataEC50: 2.00E+3nMAssay Description:Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05...More data for this Ligand-Target Pair
Affinity DataEC50: 3.50E+3nMAssay Description:Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05...More data for this Ligand-Target Pair
Affinity DataEC50: 2.20E+3nMAssay Description:Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05...More data for this Ligand-Target Pair
Affinity DataEC50: 3.08E+3nMAssay Description:Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05...More data for this Ligand-Target Pair
Affinity DataEC50: 3.15E+3nMAssay Description:Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05...More data for this Ligand-Target Pair
Affinity DataEC50: 4.40E+3nMAssay Description:Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05...More data for this Ligand-Target Pair
Affinity DataEC50: 1.64E+4nMAssay Description:Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05...More data for this Ligand-Target Pair
Affinity DataEC50: 1.81E+4nMAssay Description:Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05...More data for this Ligand-Target Pair
Affinity DataEC50: 2.10E+4nMAssay Description:Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05...More data for this Ligand-Target Pair
Affinity DataEC50: 3.36E+4nMAssay Description:Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05...More data for this Ligand-Target Pair

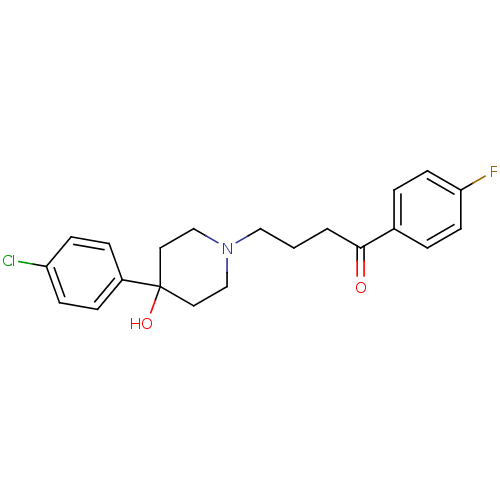
 3D Structure (crystal)
3D Structure (crystal)