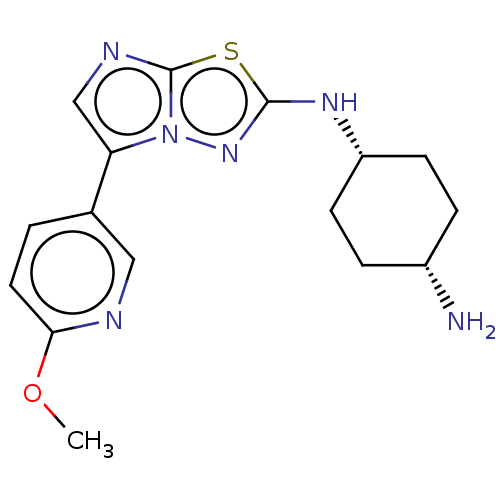
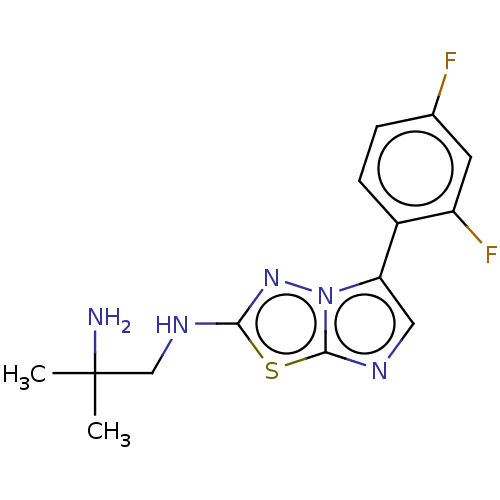
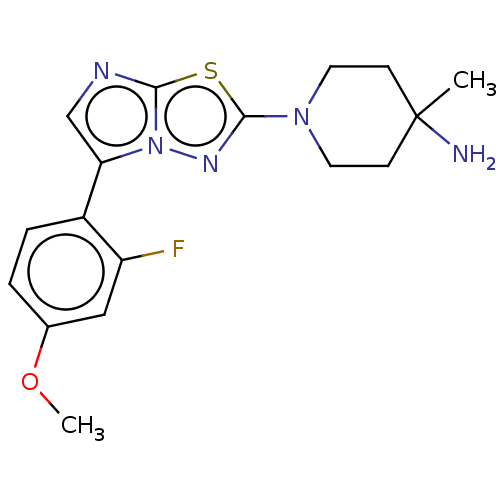
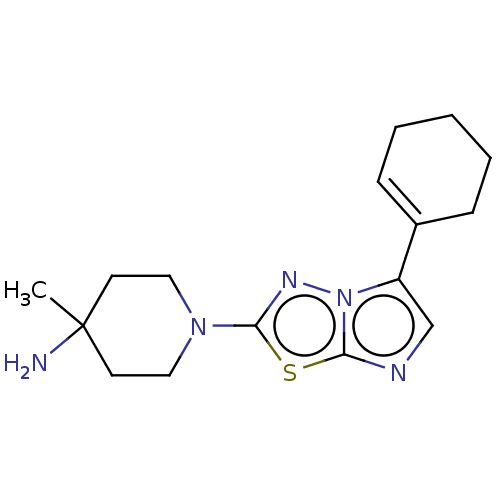
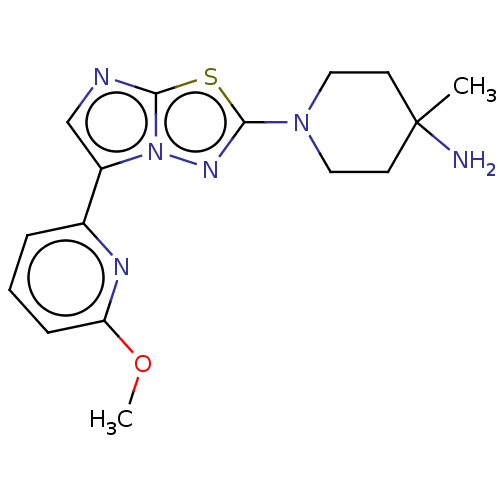
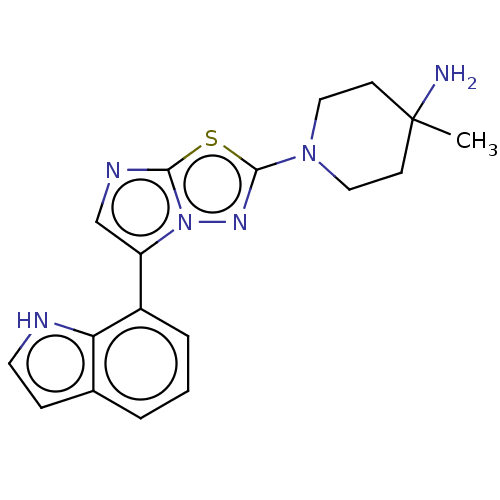
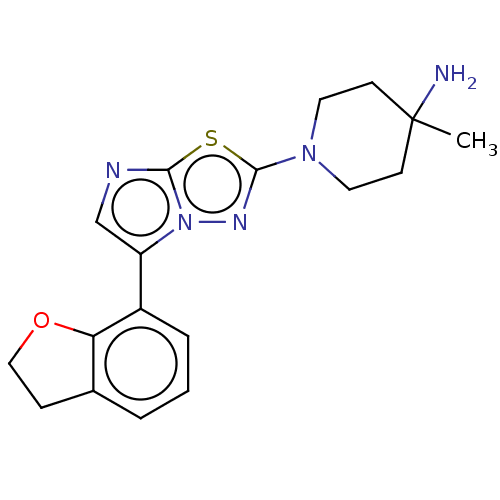
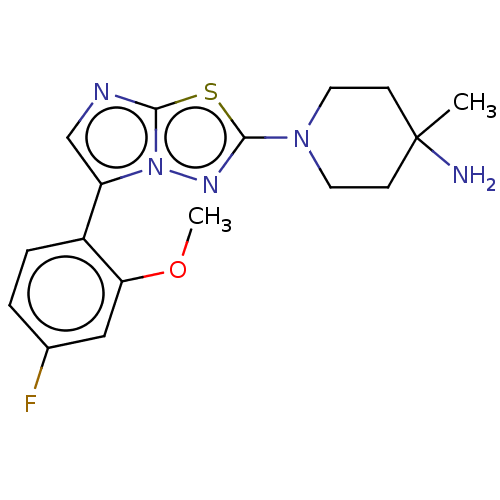
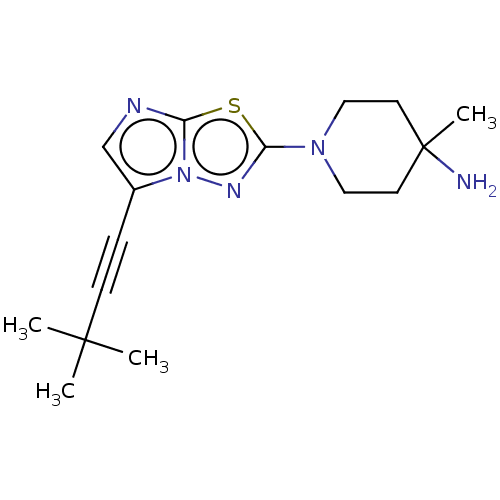
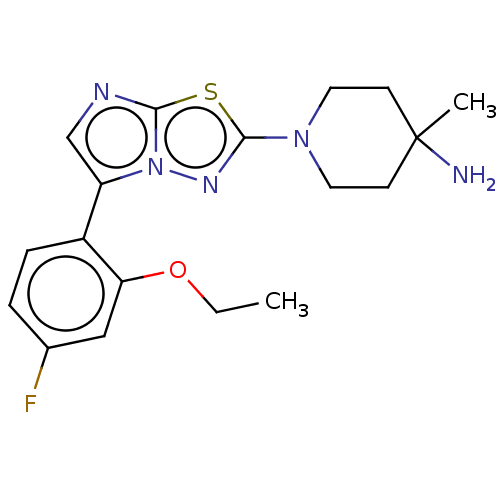
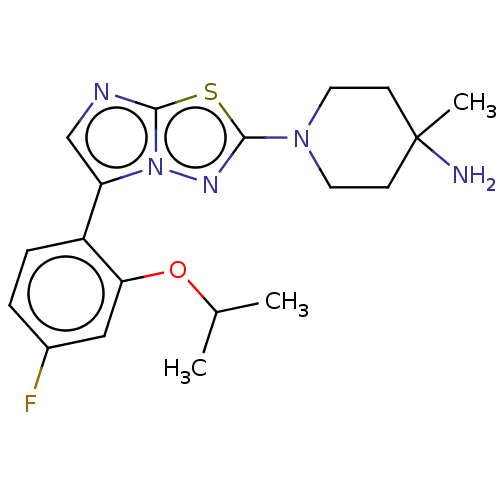
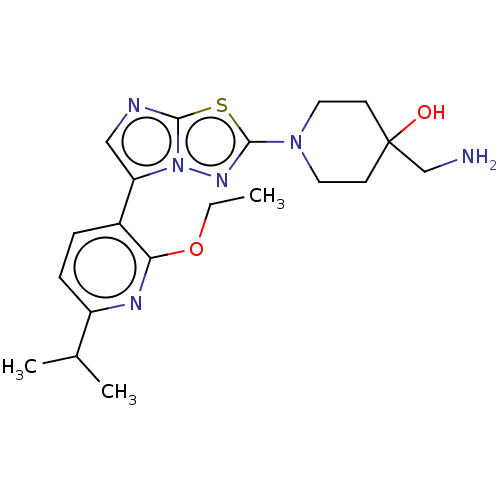
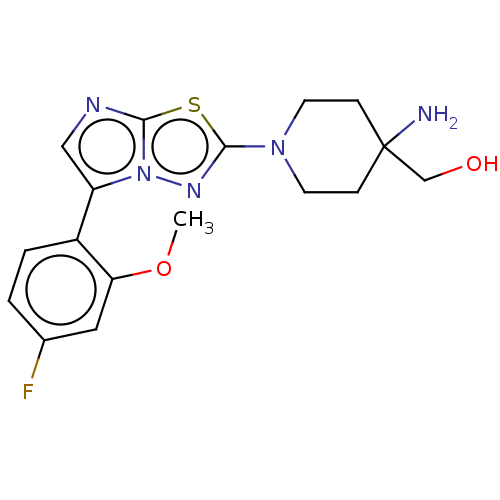
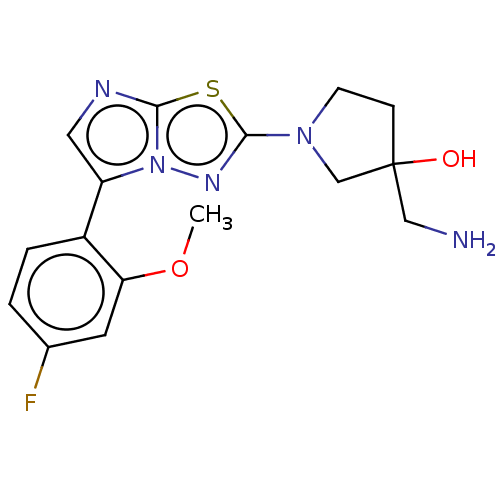
Affinity DataKi: 500nM ΔG°: -35.6kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
Affinity DataKi: 500nM ΔG°: -35.6kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
Affinity DataKi: 700nM ΔG°: -34.8kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
Affinity DataKi: 700nM ΔG°: -34.8kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+3nM ΔG°: -32.9kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
Affinity DataKi: 3.10E+3nM ΔG°: -31.1kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
Affinity DataKi: 5.00E+3nM ΔG°: -30.0kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
Affinity DataKi: 5.20E+3nM ΔG°: -29.9kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
Affinity DataKi: 5.30E+3nM ΔG°: -29.8kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
Affinity DataKi: 5.60E+3nM ΔG°: -29.7kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
Affinity DataKi: 7.70E+3nM ΔG°: -28.9kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
Affinity DataKi: 8.70E+3nM ΔG°: -28.6kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
Affinity DataKi: 8.80E+3nM ΔG°: -28.6kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
Affinity DataKi: 9.20E+3nM ΔG°: -28.5kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
Affinity DataKi: 1.02E+4nM ΔG°: -28.2kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
Affinity DataKi: 1.09E+4nM ΔG°: -28.0kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
Affinity DataKi: 2.43E+4nM ΔG°: -26.1kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
Affinity DataKi: 4.29E+4nM ΔG°: -24.7kJ/molepH: 7.4 T: 2°CAssay Description:The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 45nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 78nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 690nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 770nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.28E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.33E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.74E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of hERG by Qpatch-clamp methodMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of human FLT3More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4.04E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4.96E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5.31E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5.40E+3nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetCytochrome P450 2B6(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of CYP2B6 (unknown origin)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 8.07E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 8.50E+3nMAssay Description:Inhibition of CYP2C8 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 8.80E+3nMAssay Description:Inhibition of hERG by Qpatch-clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 8.80E+3nMAssay Description:Inhibition of hERG by Qpatch-clamp methodMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.23E+4nMAssay Description:Inhibition of hERG by Qpatch-clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.44E+4nMAssay Description:Inhibition of hERG by Qpatch-clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.44E+4nMAssay Description:Inhibition of hERG by Qpatch-clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.77E+4nMAssay Description:Inhibition of hERG by Qpatch-clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.78E+4nMAssay Description:Inhibition of hERG by Qpatch-clamp methodMore data for this Ligand-Target Pair

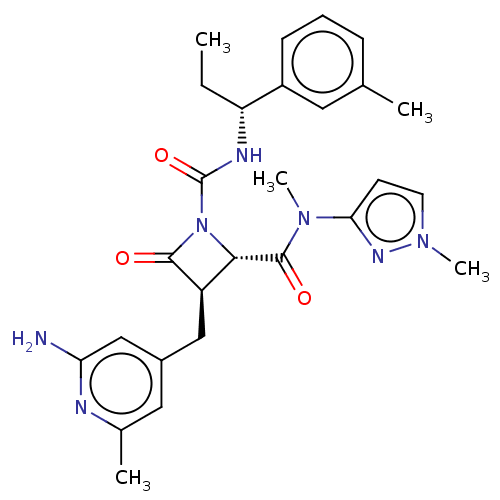
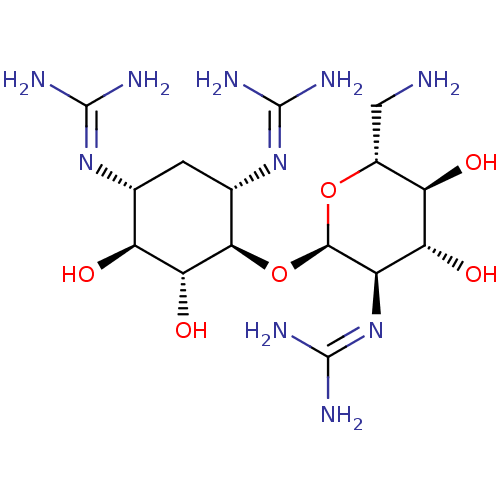
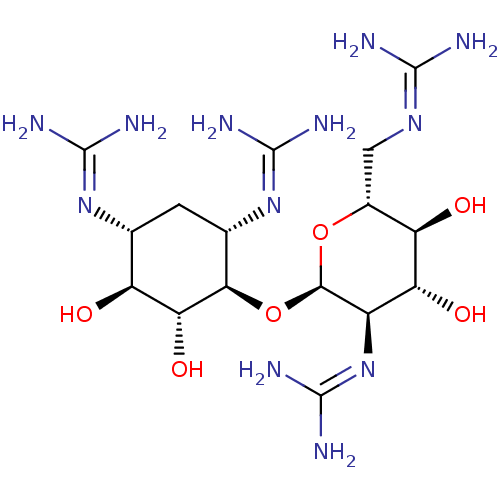
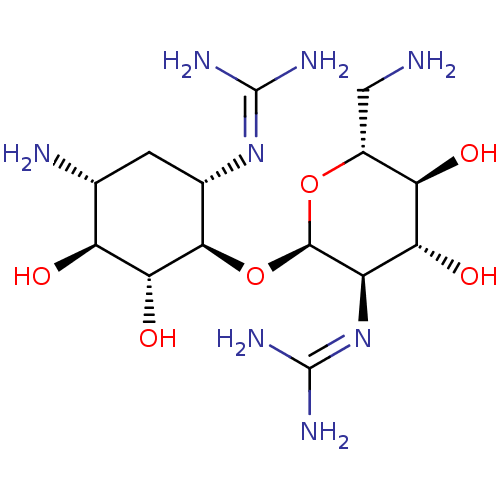
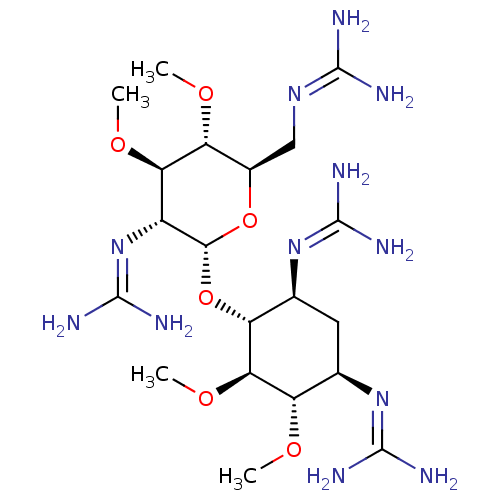
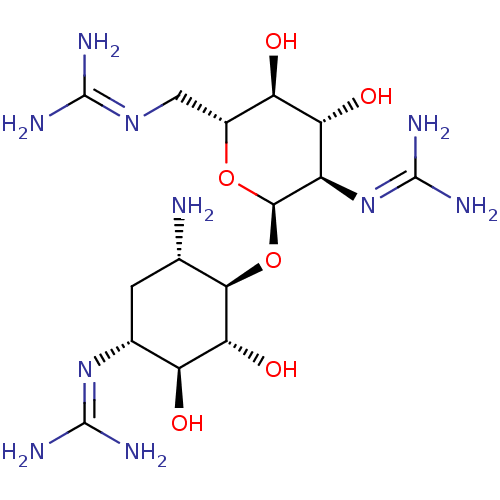
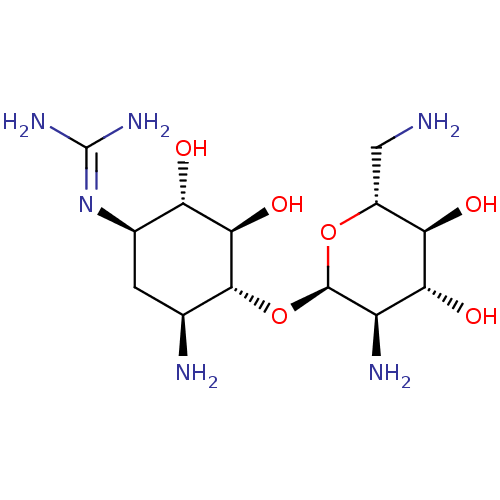
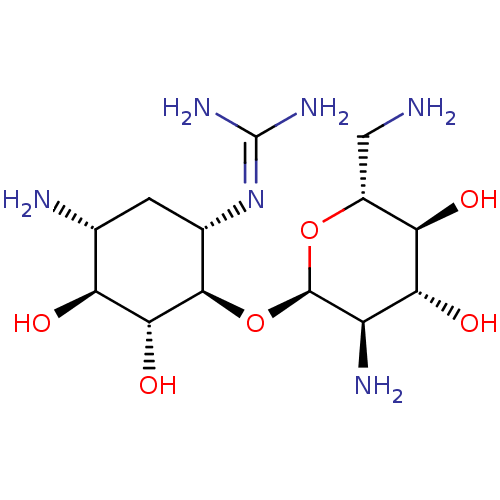
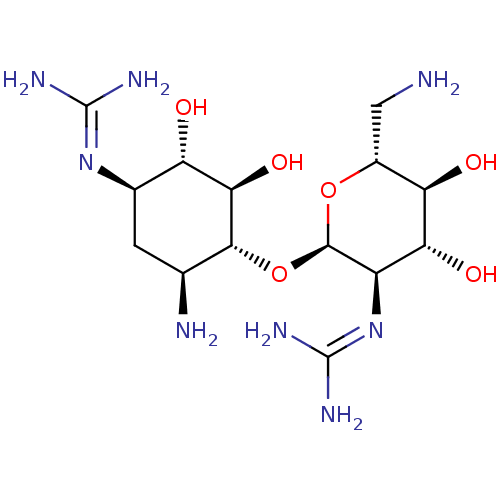
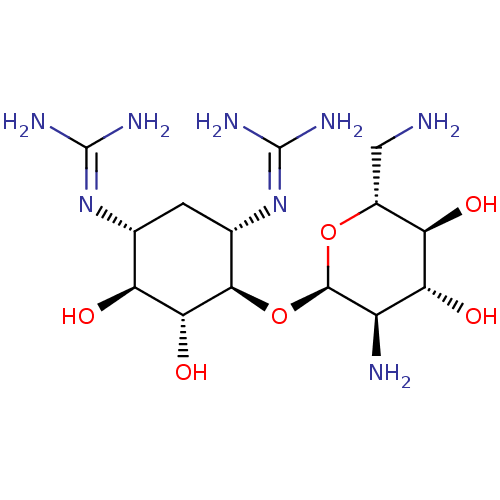
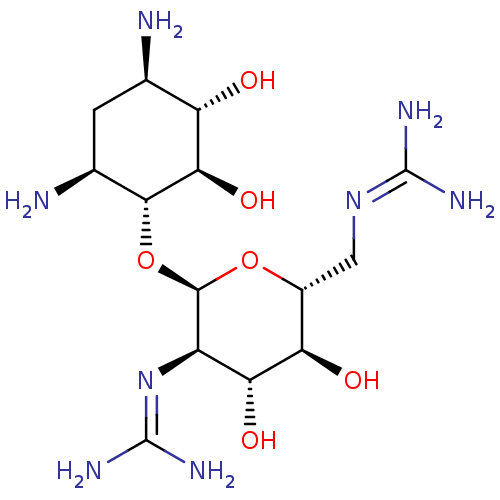
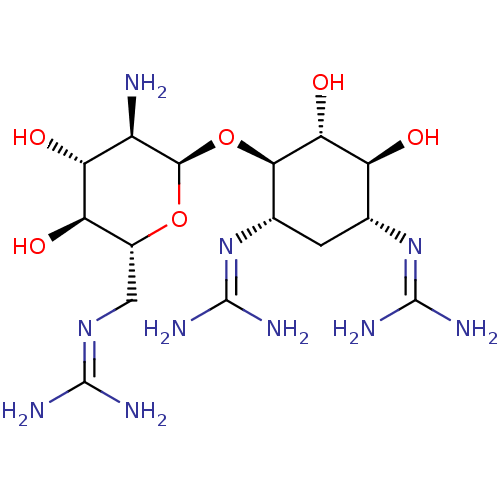
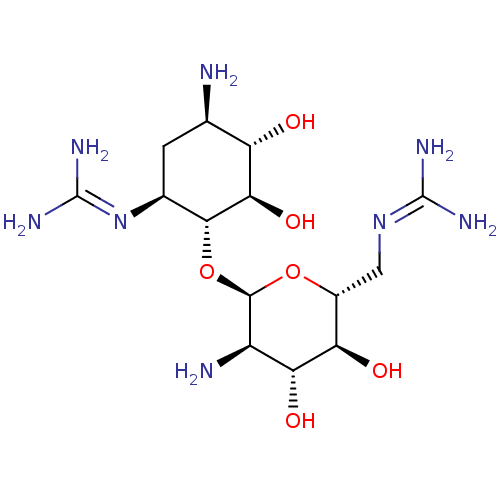
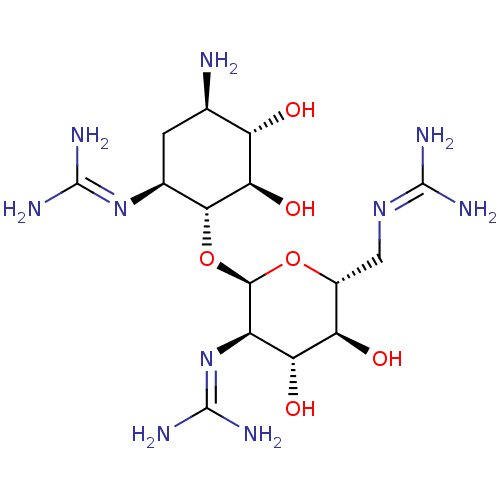
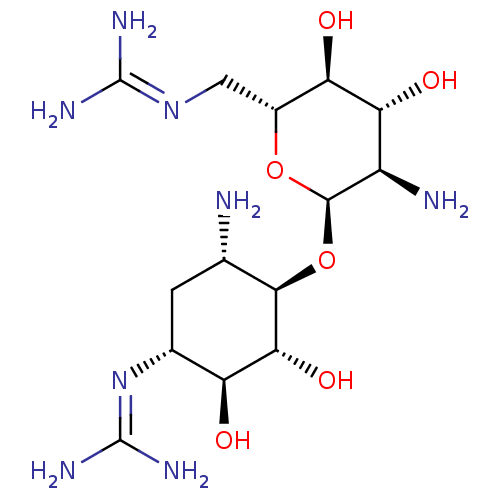
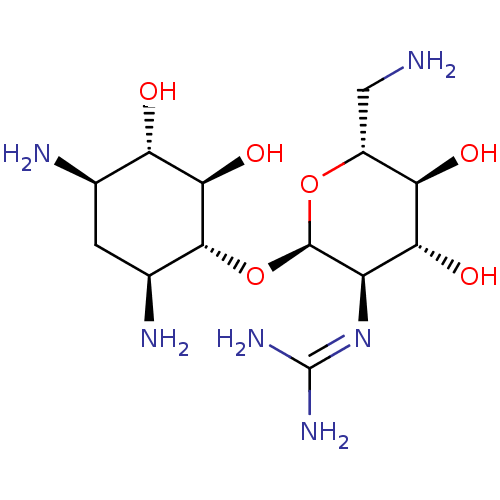
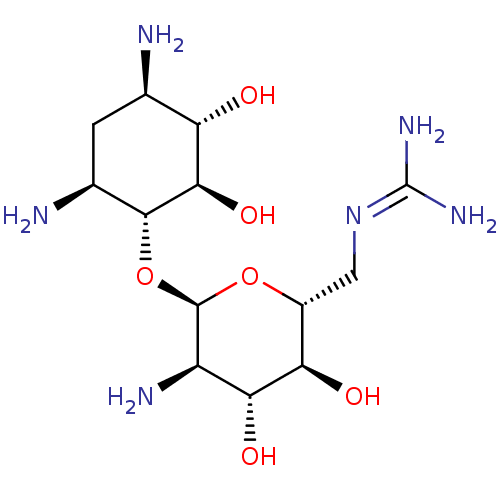
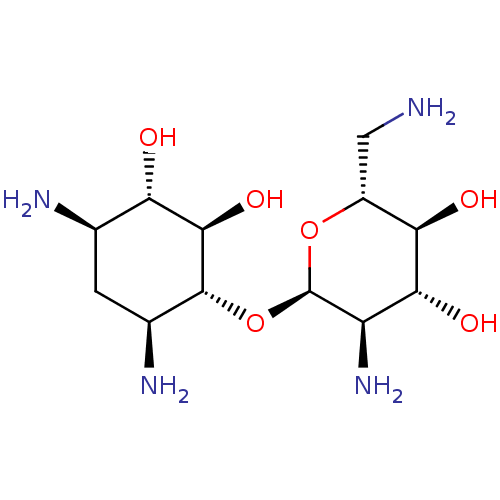

 3D Structure (crystal)
3D Structure (crystal)