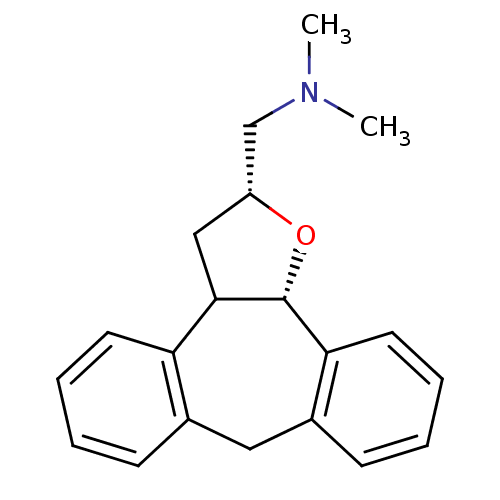
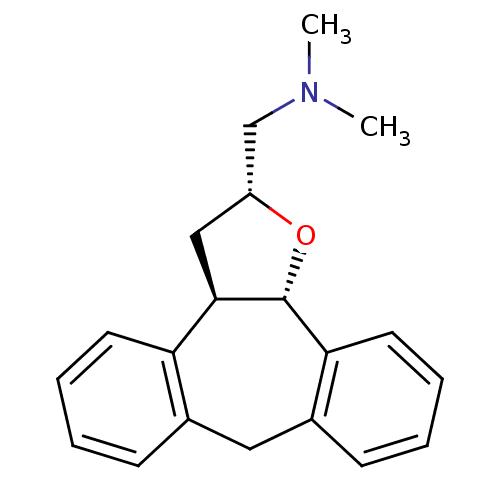
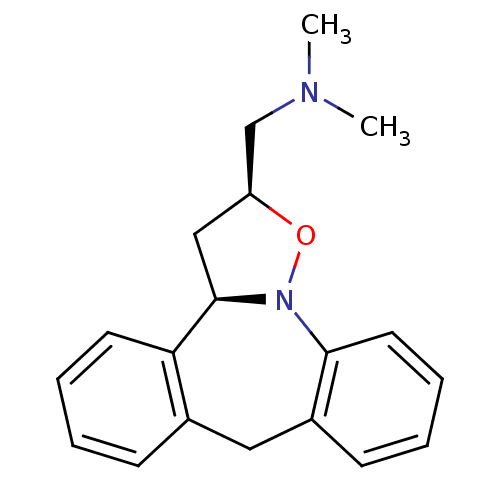
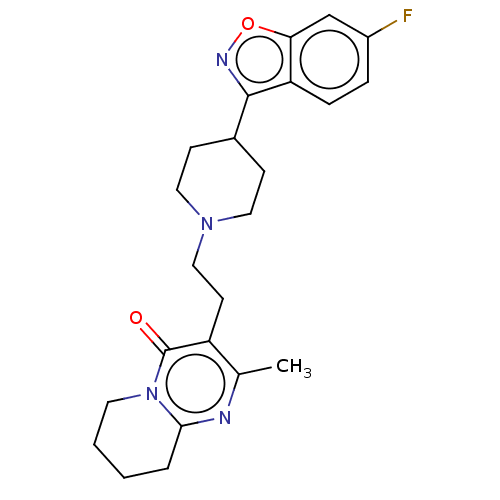
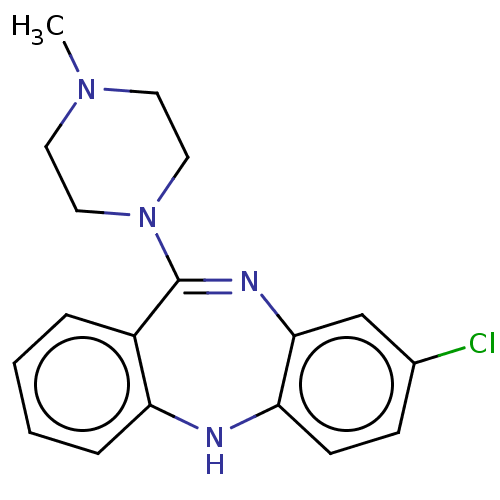
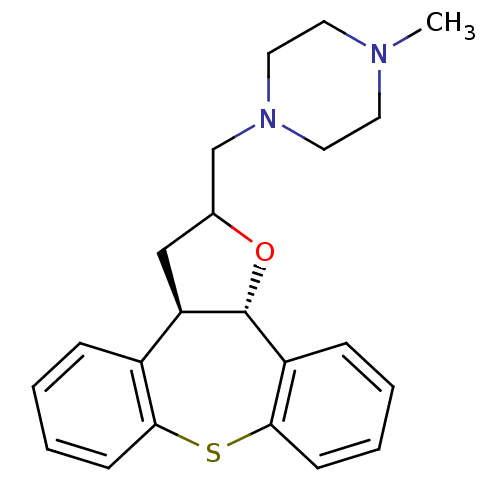
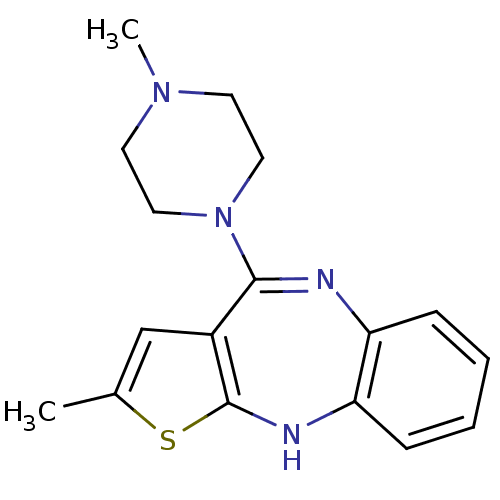
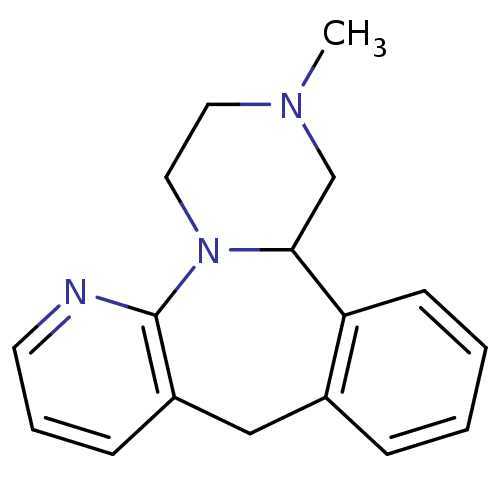
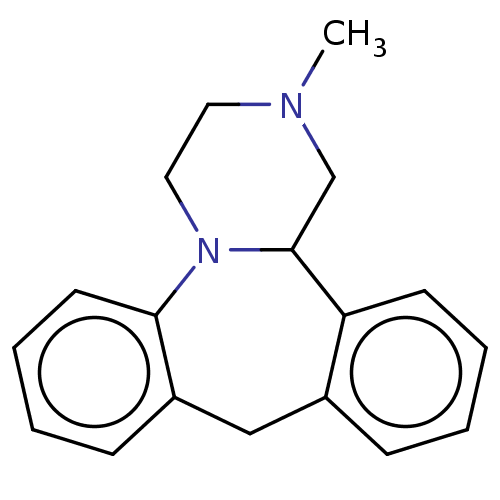
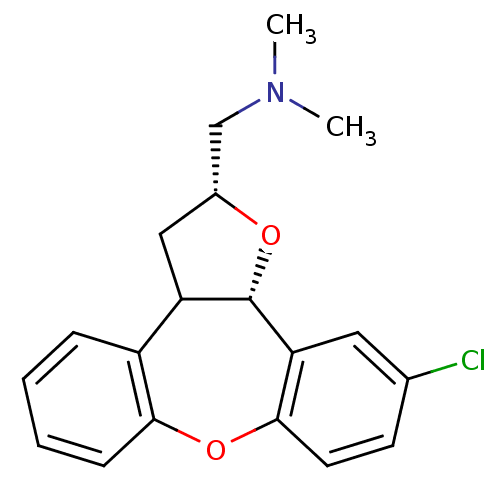
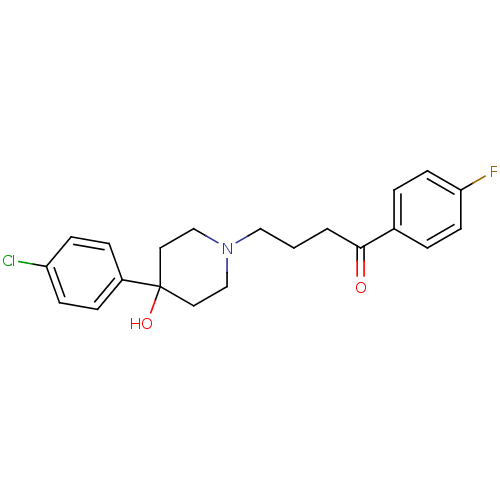
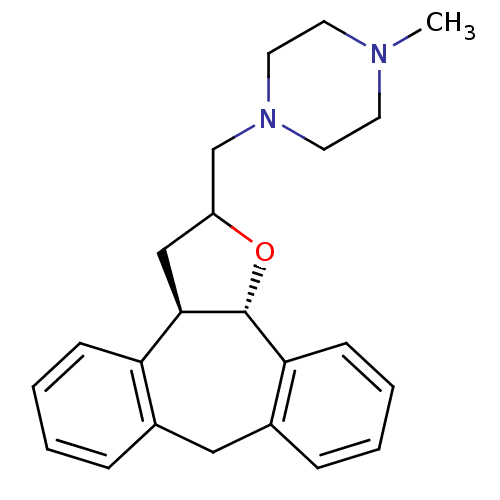
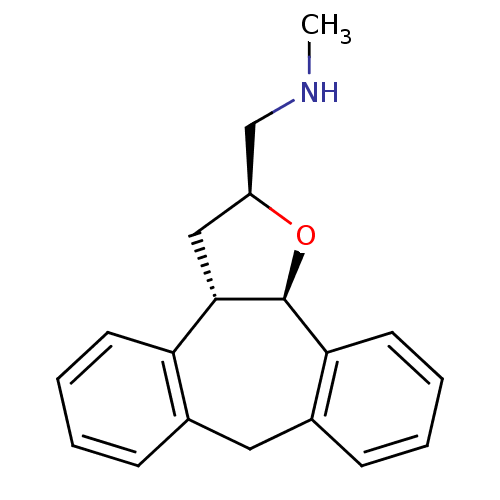
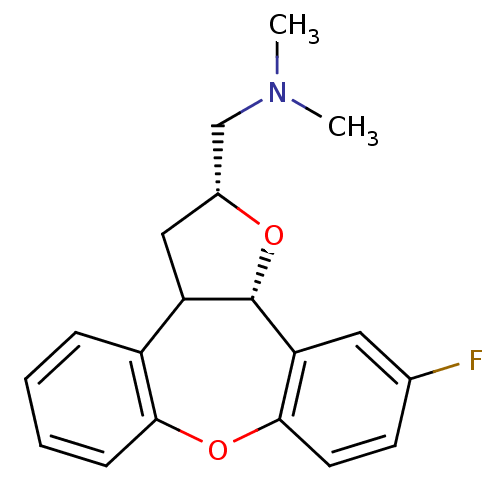
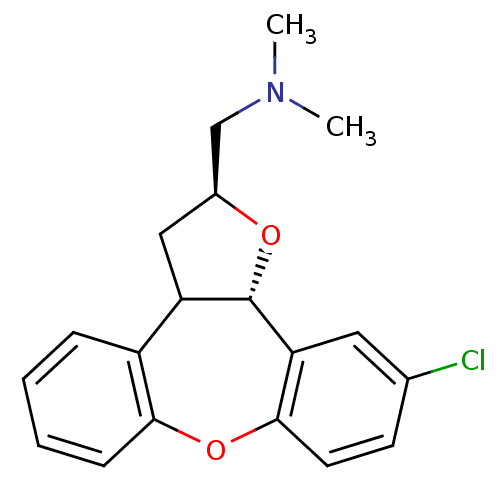
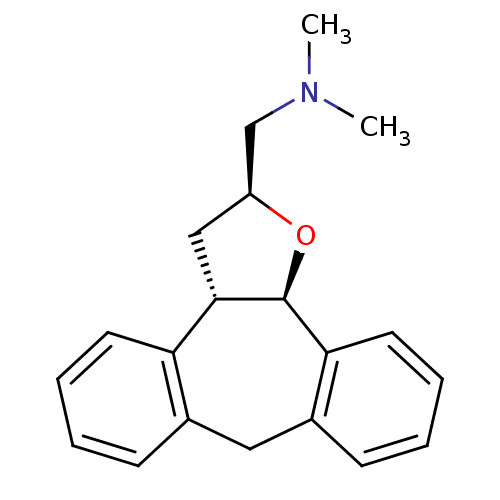
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.210nMAssay Description:Inhibition of [3H]-mesulergine binding to human 5-hydroxytryptamine 2C receptorMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.430nMAssay Description:Inhibition of [3H]-pyrilamine binding to human Histamine H1 receptor More data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.520nMAssay Description:Inhibition of [3H]-pyrilamine binding to human Histamine H1 receptor More data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.520nMAssay Description:Inhibition of [3H]-pyrilamine binding to human Histamine H1 receptor More data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.540nMAssay Description:Ability to displace [3H]-pyrilamine from human cloned histamine H1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.560nMAssay Description:Inhibition of [125I]-R91150 binding to human 5-hydroxytryptamine 2A receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.560nMAssay Description:Inhibition of [125I]-R91150 binding to human 5-hydroxytryptamine 2A receptorMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.630nMAssay Description:Ability to displace [3H]-pyrilamine from human cloned histamine H1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.640nMAssay Description:Ability to displace [3H]-pyrilamine from human cloned histamine H1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.650nMAssay Description:Inhibition of [3H]-mesulergine binding to human 5-hydroxytryptamine 2C receptorMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Ability to displace [3H]-pyrilamine from human cloned histamine H1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.810nMAssay Description:Inhibition of [125I]-R91150 binding to human 5-hydroxytryptamine 2A receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.930nMAssay Description:Inhibition of [3H]-mesulergine binding to human 5-hydroxytryptamine 2C receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.930nMAssay Description:Ability to displace [3H]- mesulergine from human cloned 5-hydroxytryptamine 2C receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Ability to displace [3H]- mesulergine from human cloned 5-hydroxytryptamine 2C receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetD(3) dopamine receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Inhibition of [125I]-iodosulpride binding to human Dopamine receptor D3More data for this Ligand-Target Pair
TargetD(3) dopamine receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Inhibition of [125I]-iodosulpride binding to human Dopamine receptor D3More data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Inhibition of [3H]-pyrilamine binding to human Histamine H1 receptor More data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Inhibition of [3H]-pyrilamine binding to human Histamine H1 receptor More data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Ability to displace [3H]-pyrilamine from human cloned histamine H1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Inhibition of [3H]-pyrilamine binding to human Histamine H1 receptor More data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Inhibition of [3H]-pyrilamine binding to human Histamine H1 receptor More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Inhibition of [3H]-mesulergine binding to human 5-hydroxytryptamine 2C receptorMore data for this Ligand-Target Pair
TargetTransporter(Rattus norvegicus)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Inhibition of [3H]-nisoxetine binding to rat Norepinephrine transpoterMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Inhibition of [125I]-R91150 binding to human 5-hydroxytryptamine 2A receptorMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Inhibition of [3H]-pyrilamine binding to human Histamine H1 receptor More data for this Ligand-Target Pair
TargetTransporter(Rattus norvegicus)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Inhibition of [3H]-nisoxetine binding to rat Norepinephrine transpoterMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Inhibition of [3H]-pyrilamine binding to human Histamine H1 receptor More data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.90nMAssay Description:Ability to displace [3H]-pyrilamine from human cloned histamine H1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetD(2) dopamine receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Inhibition of [3H]-spiperone binding to human Dopamine receptor D2More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Inhibition of [125I]-R91150 binding to human 5-hydroxytryptamine 2A receptorMore data for this Ligand-Target Pair
TargetD(2) dopamine receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Inhibition of [3H]-spiperone binding to human Dopamine receptor D2More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.40nMAssay Description:Inhibition of [3H]-mesulergine binding to human 5-hydroxytryptamine 2C receptorMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.40nMAssay Description:Ability to displace [3H]-pyrilamine from human cloned histamine H1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Ability to displace [125I]-R91150 from human cloned 5-hydroxytryptamine 2A receptor expressed in L929 cellsMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Inhibition of [125I]-R91150 binding to human 5-hydroxytryptamine 2A receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Ability to displace [125I]-R91150 from human cloned 5-hydroxytryptamine 2A receptor expressed in L929 cellsMore data for this Ligand-Target Pair
TargetD(2) dopamine receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Inhibition of [3H]-spiperone binding to human Dopamine receptor D2More data for this Ligand-Target Pair
TargetTransporter(Rattus norvegicus)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.70nMAssay Description:Inhibition of [3H]-nisoxetine binding to rat Norepinephrine transpoterMore data for this Ligand-Target Pair
TargetAlpha-2C adrenergic receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.90nMAssay Description:Inhibition of [3H]-rauwolscine binding to Alpha-2C adrenergic receptorMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Ability to displace [3H]-pyrilamine from human cloned histamine H1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAlpha-2C adrenergic receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Inhibition of [3H]-rauwolscine binding to Alpha-2C adrenergic receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.30nMAssay Description:Ability to displace [3H]- mesulergine from human cloned 5-hydroxytryptamine 2C receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.5nMAssay Description:Ability to displace [3H]-pyrilamine from human cloned histamine H1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetTransporter(Rattus norvegicus)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.60nMAssay Description:Inhibition of [3H]-nisoxetine binding to rat Norepinephrine transpoterMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Inhibition of [3H]-mesulergine binding to human 5-hydroxytryptamine 2C receptorMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Ability to displace [3H]-pyrilamine from human cloned histamine H1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Ability to displace [3H]-pyrilamine from human cloned histamine H1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAlpha-2C adrenergic receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.80nMAssay Description:Inhibition of [3H]-rauwolscine binding to Alpha-2C adrenergic receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4.30nMAssay Description:Inhibition of [125I]-R91150 binding to human 5-hydroxytryptamine 2A receptorMore data for this Ligand-Target Pair