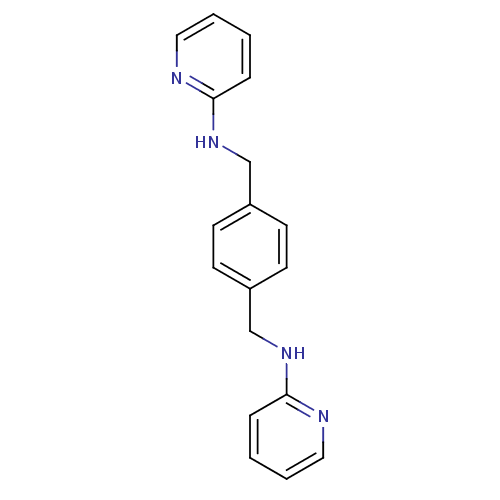
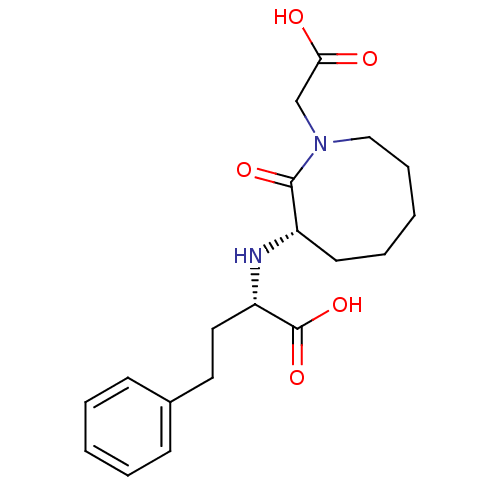
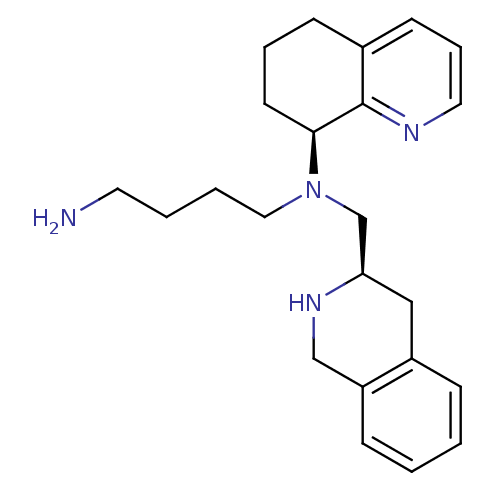
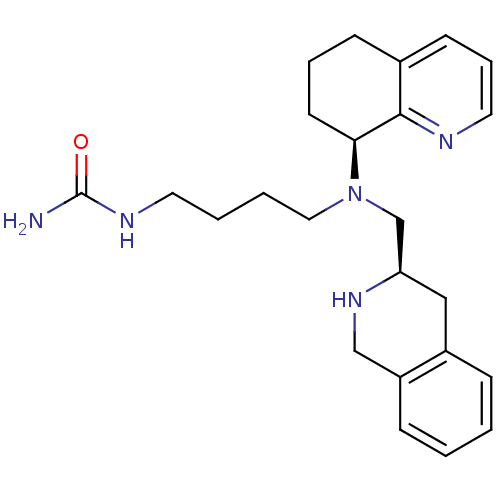
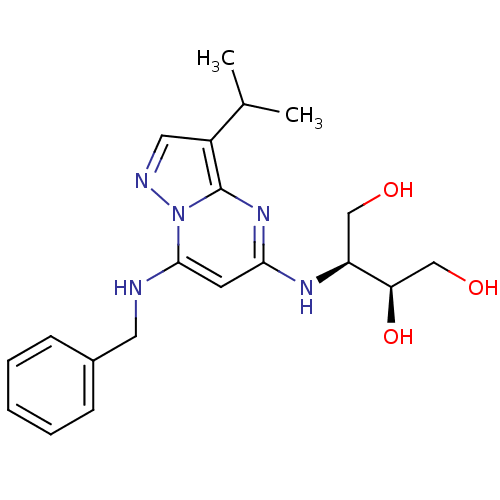
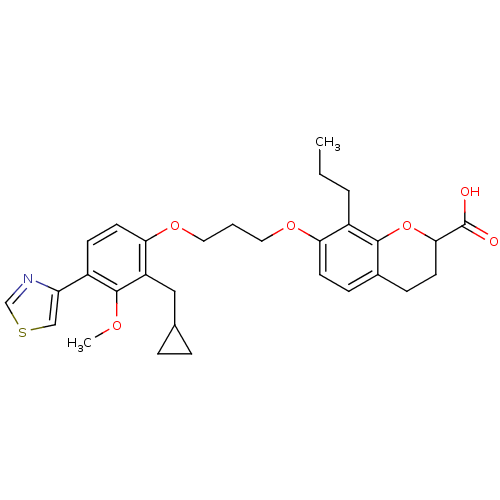
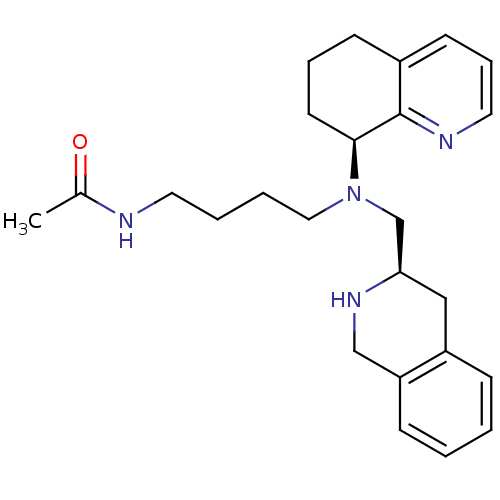
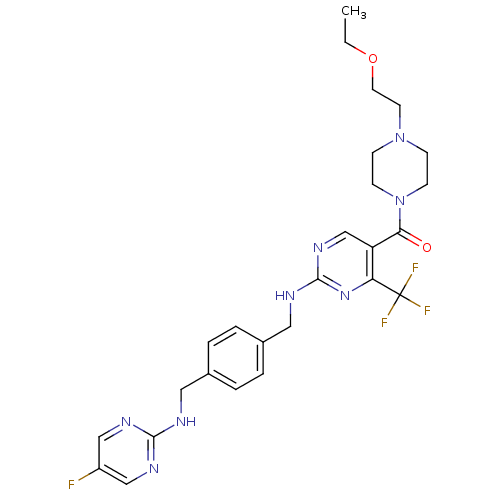
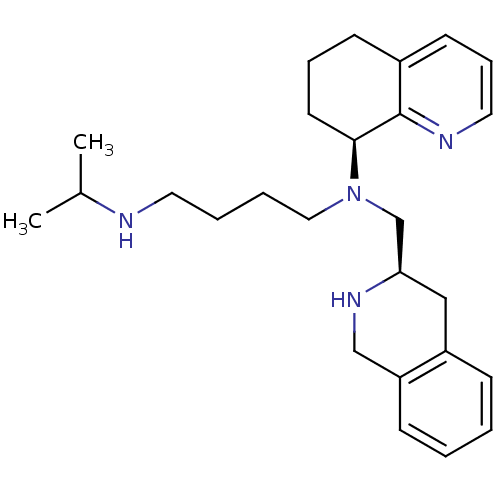
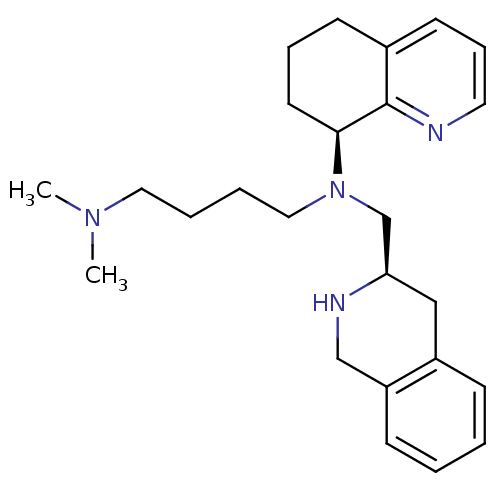
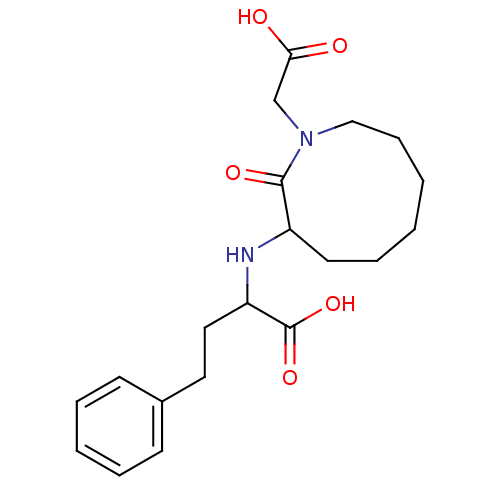
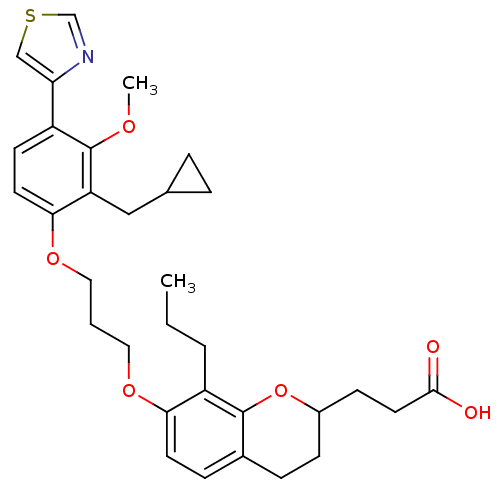
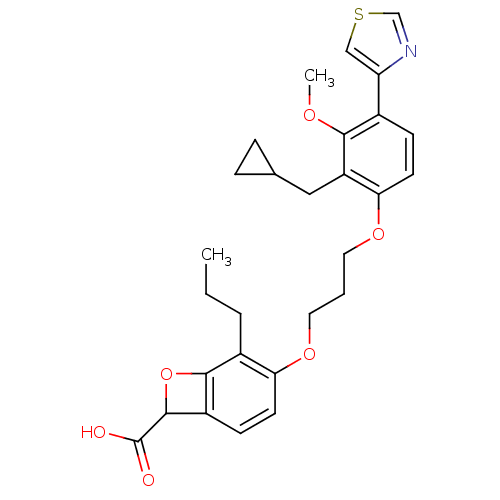
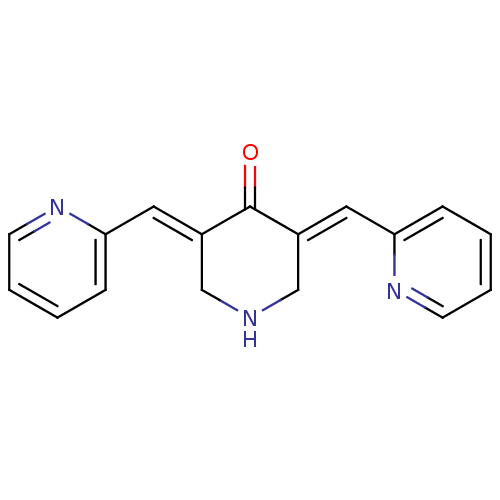
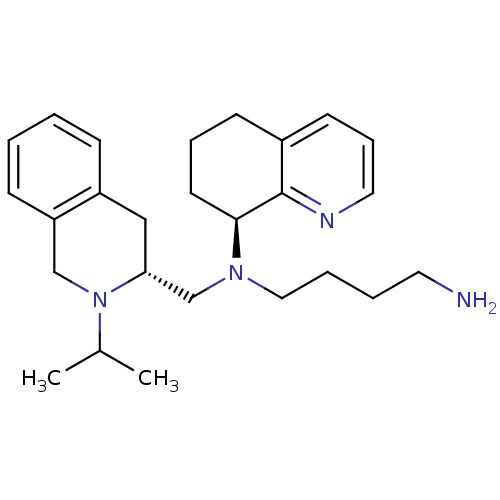
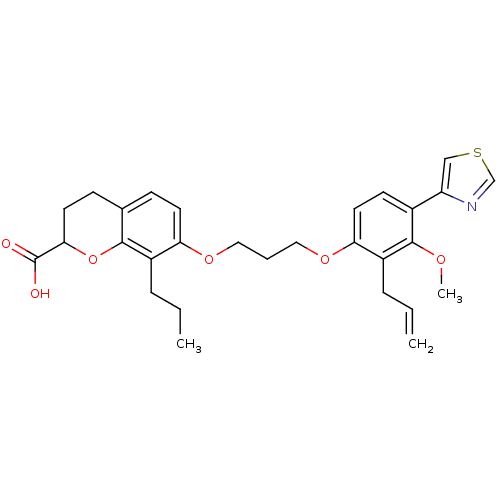
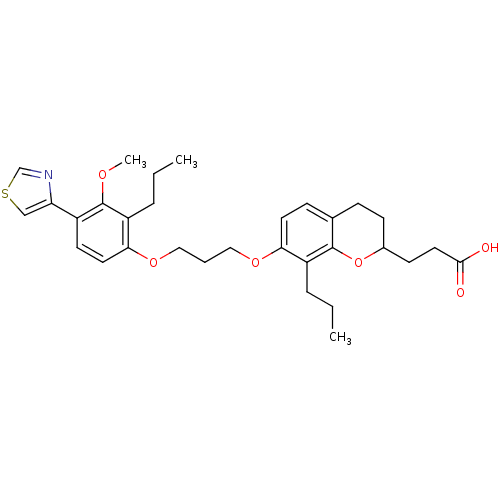
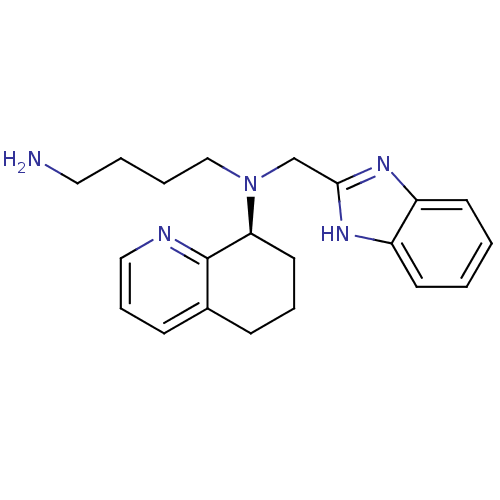
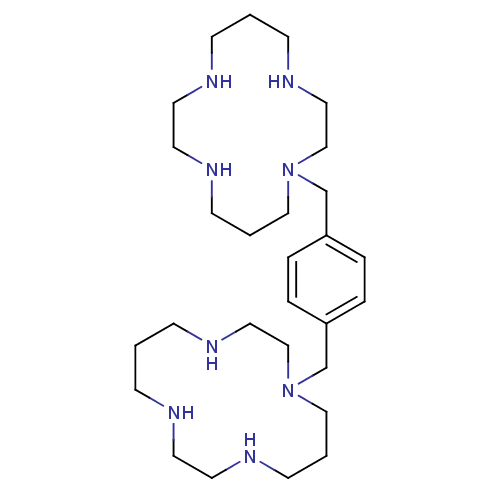
Affinity DataKi: 0.300nMAssay Description:Inhibition of carbonic anhydrase-2 (unknown origin) incubated for 15 mins prior to testing by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.770nMAssay Description:Inhibition of carbonic anhydrase-2 (unknown origin) incubated for 15 mins prior to testing by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Inhibition of carbonic anhydrase-2 (unknown origin) incubated for 15 mins prior to testing by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.70nMAssay Description:Inhibition of carbonic anhydrase-2 (unknown origin) incubated for 15 mins prior to testing by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:Inhibition of carbonic anhydrase-2 (unknown origin) incubated for 15 mins prior to testing by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
US Patent
Emory University
US Patent
Affinity DataKi: 553nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
US Patent
Emory University
US Patent
Affinity DataKi: 1.00E+3nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
US Patent
Emory University
US Patent
Affinity DataKi: 1.60E+3nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
US Patent
Emory University
US Patent
Affinity DataKi: 5.00E+3nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
US Patent
Emory University
US Patent
Affinity DataKi: 7.50E+3nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
US Patent
Emory University
US Patent
Affinity DataKi: 7.50E+3nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
US Patent
Emory University
US Patent
Affinity DataKi: 7.50E+3nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of histamine H2 receptor (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
US Patent
Emory University
US Patent
Affinity DataKi: 1.00E+4nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
US Patent
Emory University
US Patent
Affinity DataKi: 1.30E+4nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
US Patent
Emory University
US Patent
Affinity DataKi: 3.90E+4nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of CXCR4 in MDA-MB-231 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Antagonist activity at human CXCR4 expressed in human U87 cells expressing CD4 assessed as inhibition of CXCL12-induced cAMP production pretreated fo...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibitory concentration against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Antagonist activity at human CXCR4 expressed in human U87 cells expressing CD4 assessed as inhibition of CXCL12-induced cAMP production pretreated fo...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibitory concentration (isomer B) against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assayMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Imperial College
Curated by ChEMBL
Imperial College
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of CDK2/cyclinE assessed as amount of ATP released by luciferase activity based PKLight assayMore data for this Ligand-Target Pair
TargetLeukotriene B4 receptor 1(Homo sapiens (Human))
Searle Research And Development
Curated by ChEMBL
Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Binding affinity of the compound towards Leukotriene B4 (LTB4) Receptor. Experiment conducted in the absence of NDGA.More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMAssay Description:Inhibitory concentration (isomer B) against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ...More data for this Ligand-Target Pair
TargetLeukotriene B4 receptor 1(Homo sapiens (Human))
Searle Research And Development
Curated by ChEMBL
Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 5.20nMAssay Description:Binding affinity of the compound towards Leukotriene B4 (LTB4) Receptor. Experiment conducted in the absence of NDGA.More data for this Ligand-Target Pair
Affinity DataIC50: 5.40nMAssay Description:Antagonist activity at human CXCR4 expressed in human U87 cells expressing CD4 assessed as inhibition of CXCL12-induced cAMP production pretreated fo...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8.10nMAssay Description:Inhibitory concentration (isomer B) against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetLeukotriene B4 receptor 1(Homo sapiens (Human))
Searle Research And Development
Curated by ChEMBL
Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 12.3nMAssay Description:Binding affinity of the compound towards Leukotriene B4 (LTB4) Receptor. Experiment conducted in the absence of NDGA.More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Antagonist activity at CXCR4 (unknown origin) assessed as inhibition of SDF-1-induced beta-arrestin recruitment incubated for 30 mins prior to SDF-1 ...More data for this Ligand-Target Pair
TargetLeukotriene B4 receptor 1(Homo sapiens (Human))
Searle Research And Development
Curated by ChEMBL
Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 15.5nMAssay Description:Binding affinity of the compound towards Leukotriene B4 (LTB4) Receptor. Experiment conducted in the absence of NDGA.More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibitory concentration (isomer B) against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Antagonist activity at CXCR4 (unknown origin) expressed in CHO-K1 cells assessed as inhibition of SDF-1alpha/forskolin-induced cAMP productionMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ...More data for this Ligand-Target Pair
TargetRAC-beta serine/threonine-protein kinase(Homo sapiens (Human))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of recombinant AKT2 (unknown origin) by FRET-based Z'-LYTE assayMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ...More data for this Ligand-Target Pair
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of recombinant AKT1 (unknown origin) by FRET-based Z'-LYTE assayMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of CDK7/cyclinH/MAT1 assessed as amount of ATP released by luciferase activity based PKLight assayMore data for this Ligand-Target Pair
TargetLeukotriene B4 receptor 1(Homo sapiens (Human))
Searle Research And Development
Curated by ChEMBL
Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Binding affinity for Leukotriene B4 (LTB4) receptorMore data for this Ligand-Target Pair
TargetLeukotriene B4 receptor 1(Homo sapiens (Human))
Searle Research And Development
Curated by ChEMBL
Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Binding affinity for Leukotriene B4 (LTB4) receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Antagonist activity at CXCR4 in human PBMC assessed as inhibition of HIV-1 3B infectionMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Antagonist activity at CXCR4 in human PBMC assessed as inhibition of HIV-1 3B infectionMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Imperial College
Curated by ChEMBL
Imperial College
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:Inhibition of CDK1/cyclinB1 assessed as amount of ATP released by luciferase activity based PKLight assayMore data for this Ligand-Target Pair






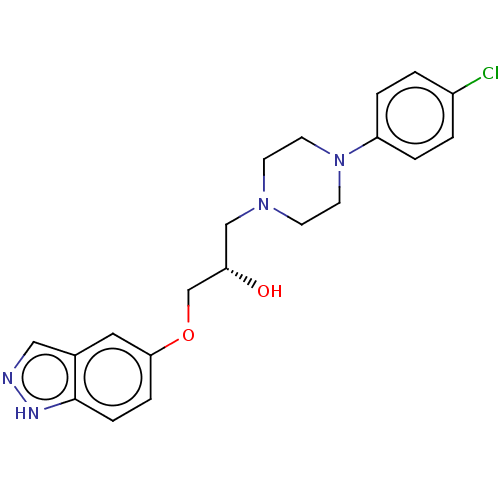

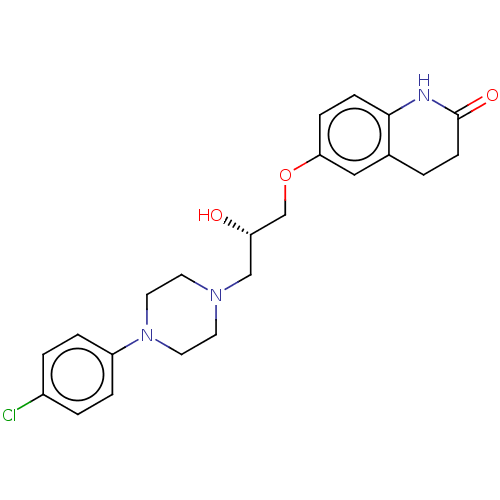
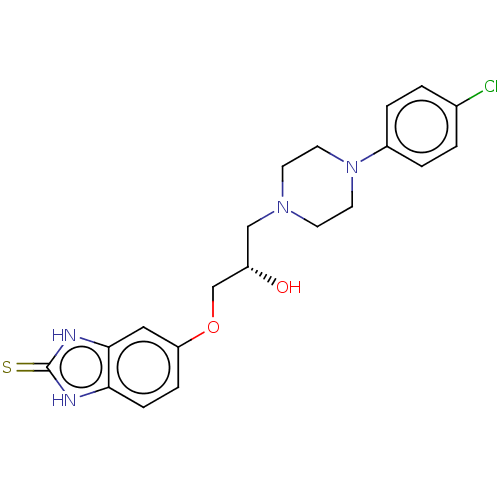
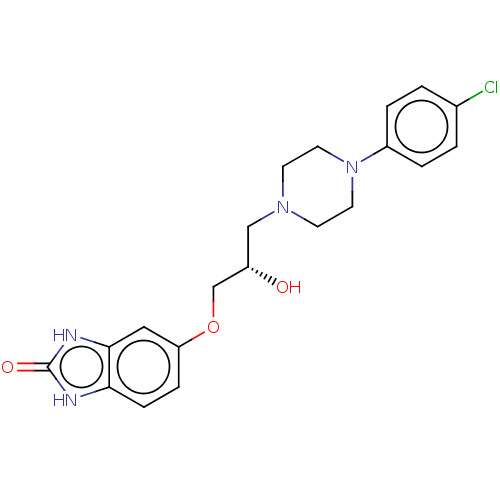
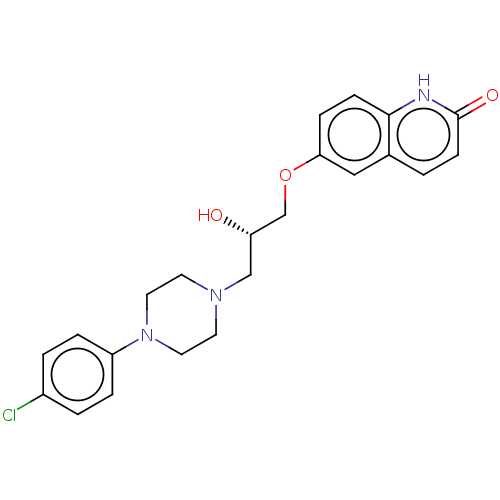
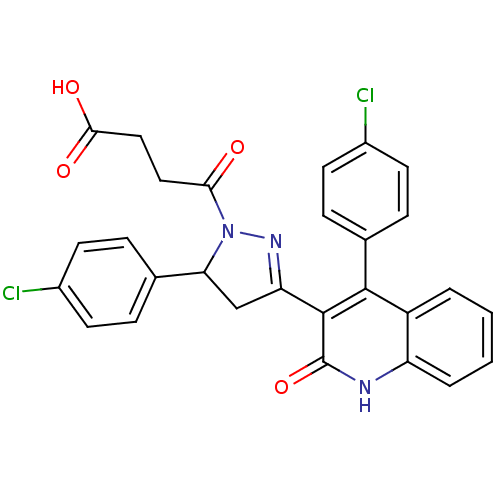
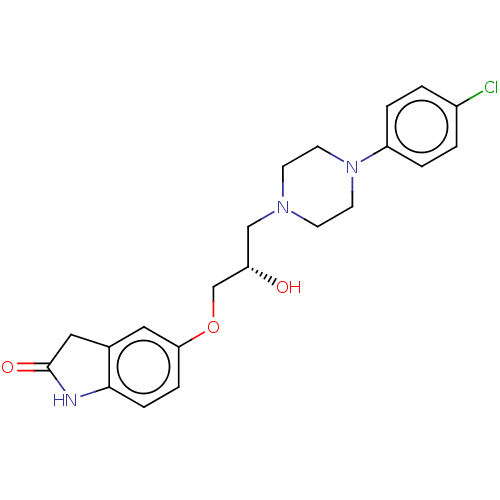
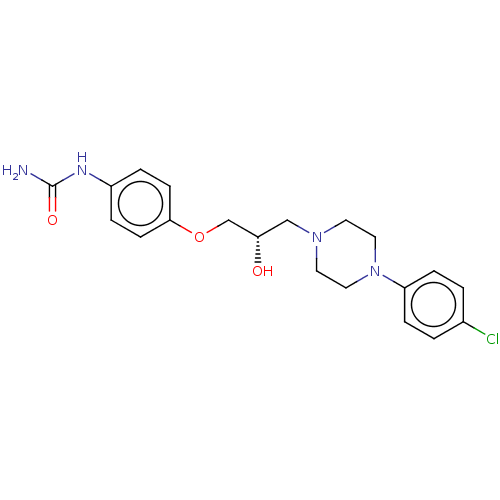
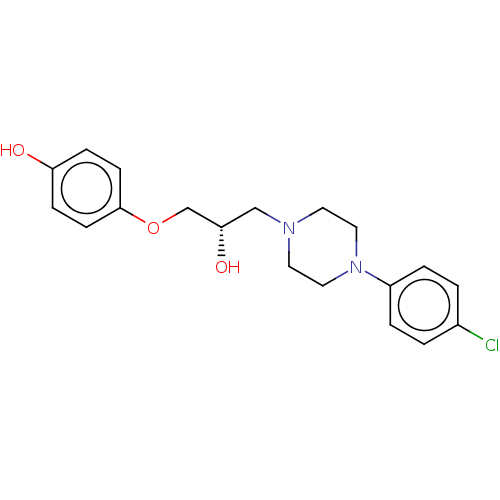
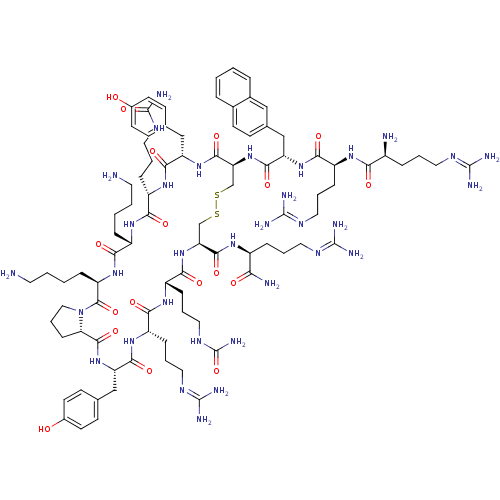
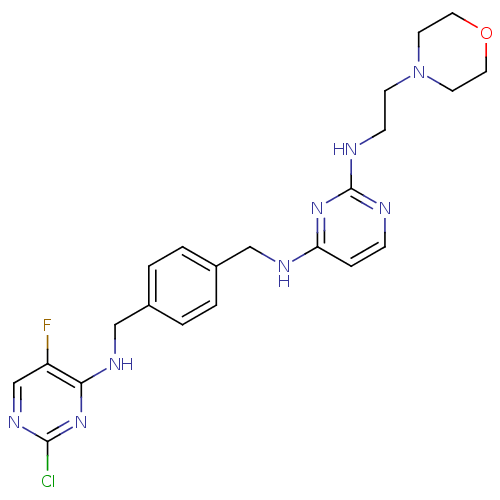
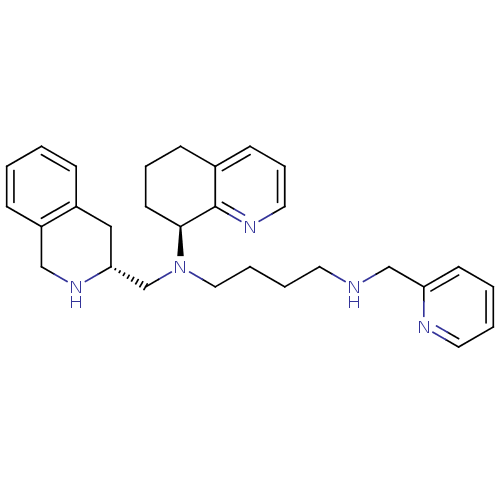
 3D Structure (crystal)
3D Structure (crystal)