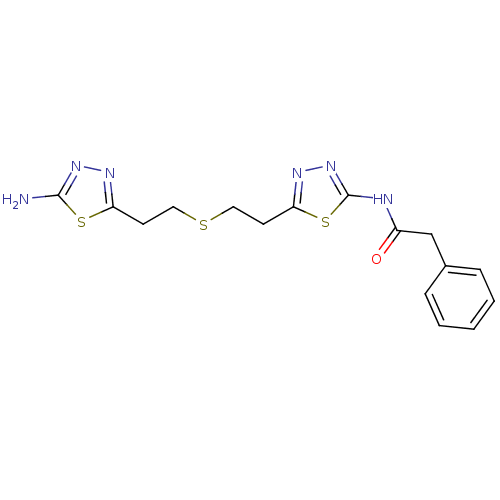
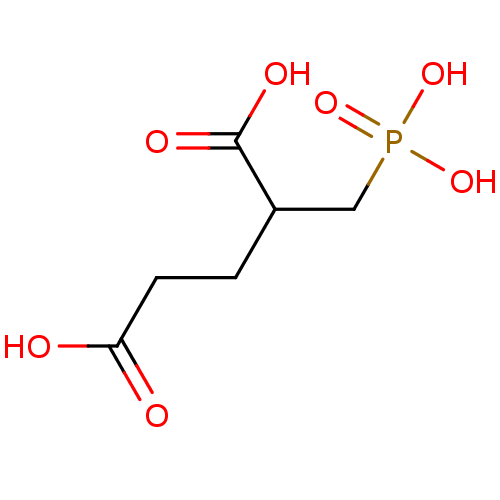
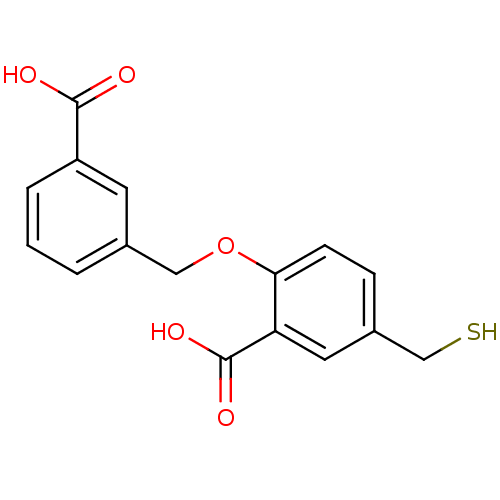
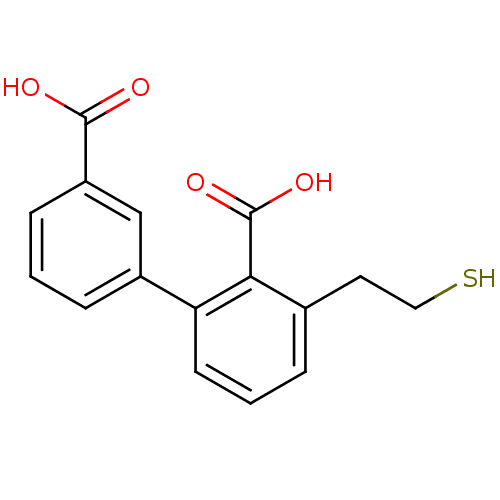
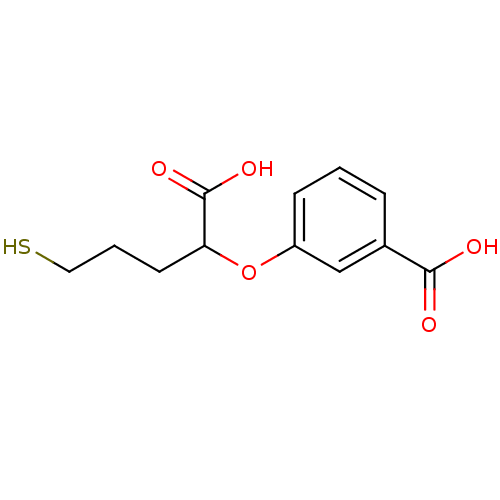
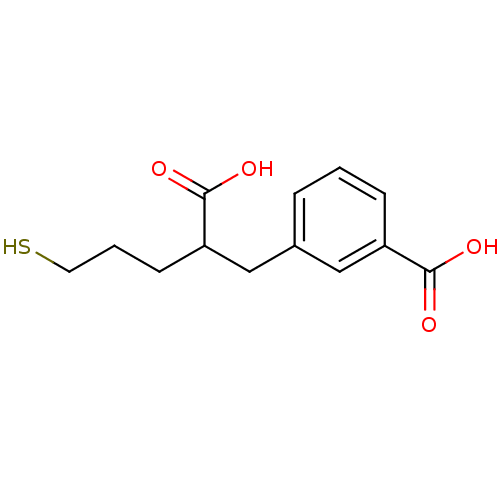
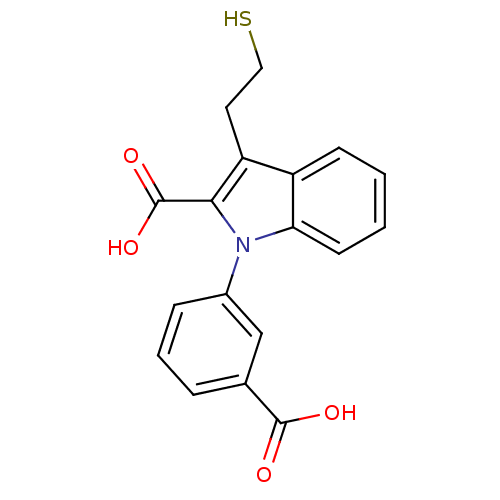
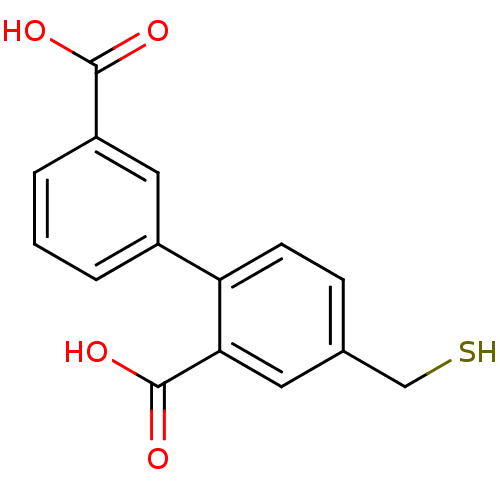
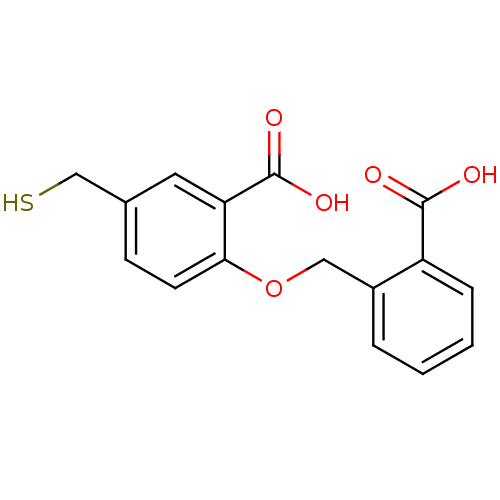
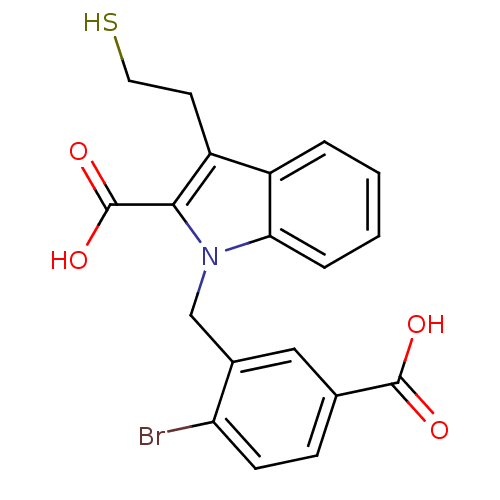
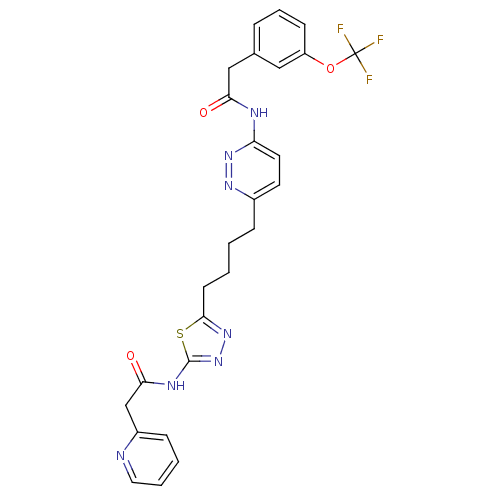
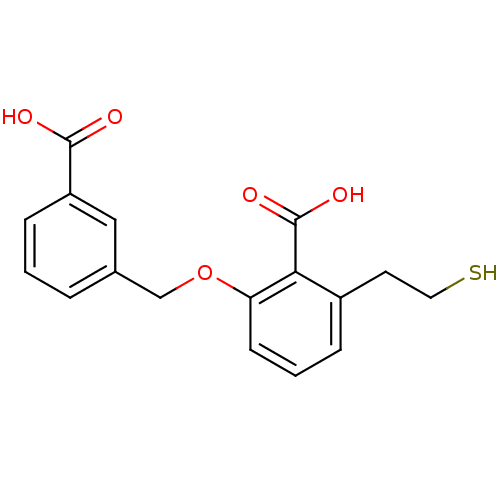
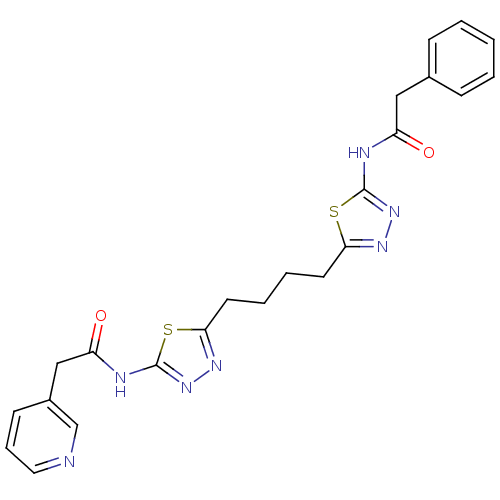
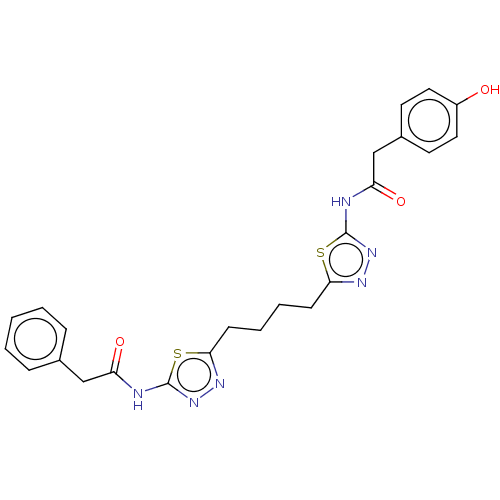
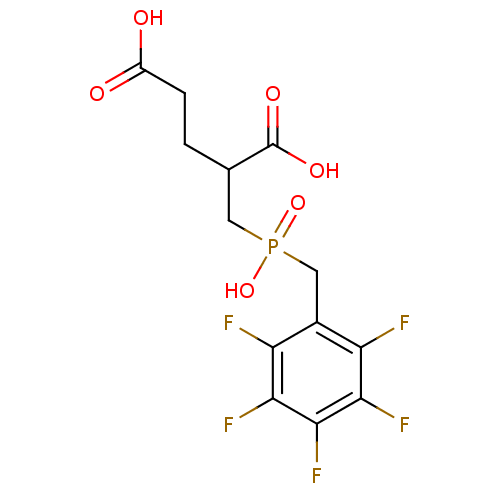
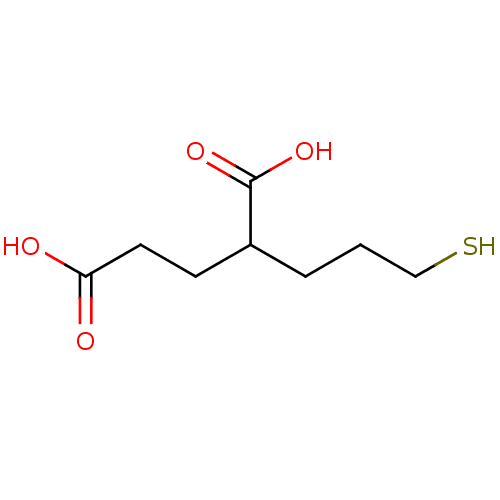
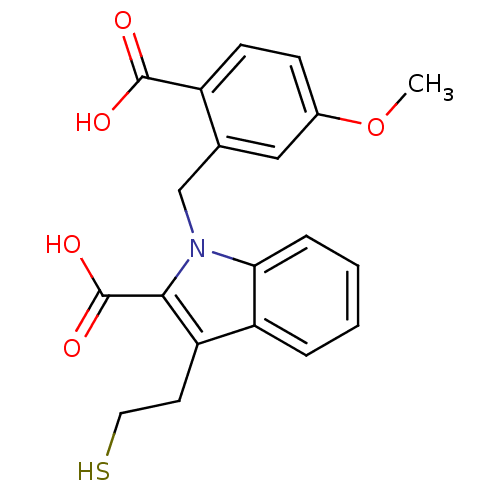
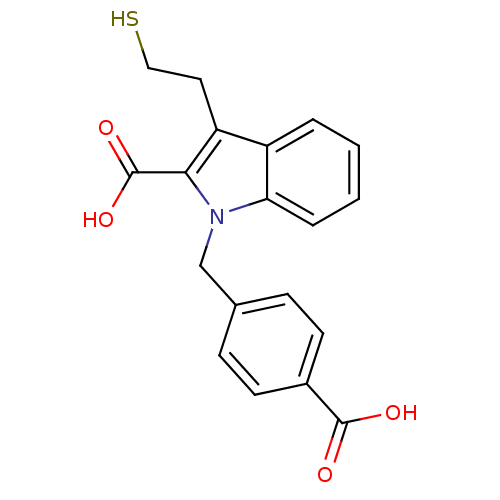
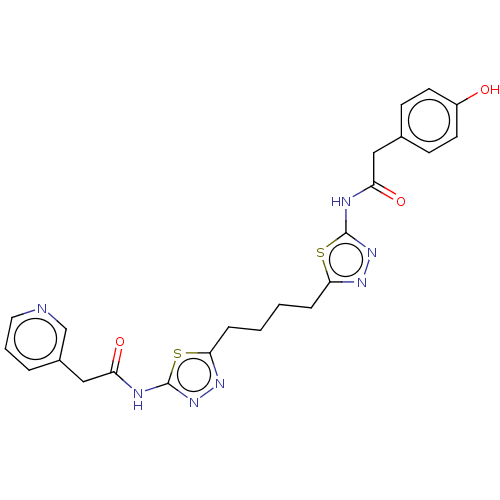
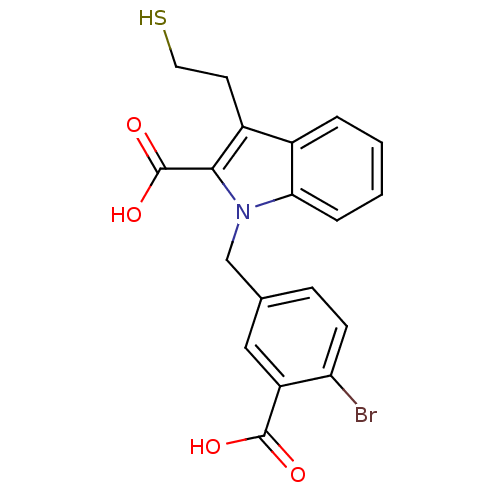
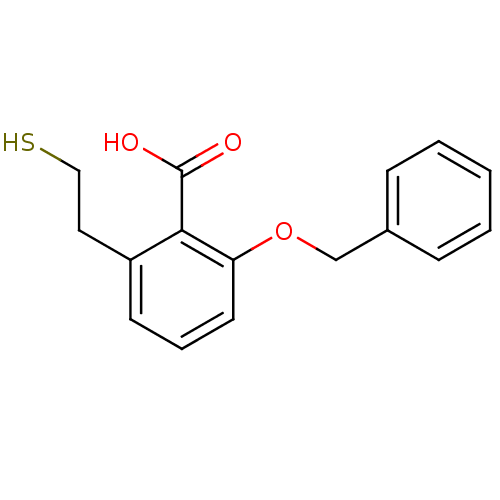
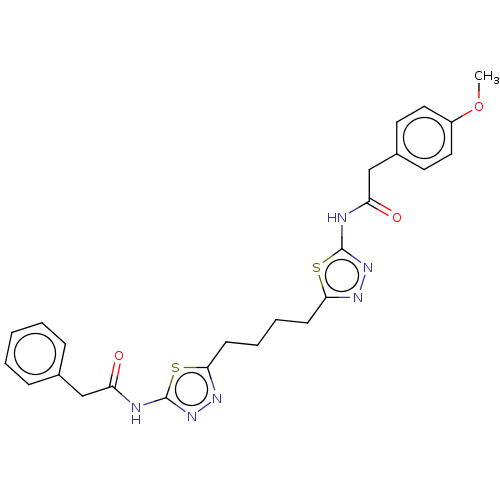
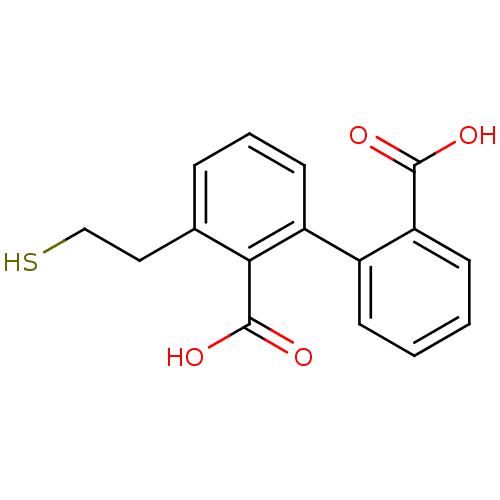
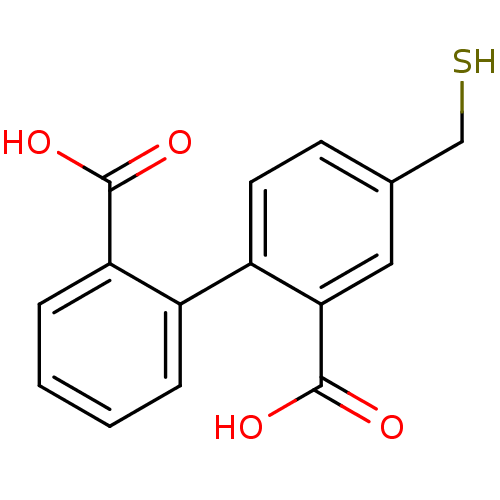
Affinity DataKi: 0.0100nMAssay Description:Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]GMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Uncompetitive inhibition of human kidney glutaminase (124 to 669) assessed as reduction of glutamine hydrolysis by double-reciprocal plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMpH: 7.4 T: 2°CAssay Description:GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMpH: 7.4 T: 2°CAssay Description:GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMpH: 7.4 T: 2°CAssay Description:GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]GMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]GMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]GMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]GMore data for this Ligand-Target Pair
Affinity DataIC50: 32nMpH: 7.4 T: 2°CAssay Description:GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ...More data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]GMore data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 52nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 54nMAssay Description:Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]GMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Inhibition of human kidney type glutaminase (124 to 669 residues) using L-[3H]-glutamine as substrate after 45 mins by topcount methodMore data for this Ligand-Target Pair
Affinity DataIC50: 63nMpH: 7.4 T: 2°CAssay Description:GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ...More data for this Ligand-Target Pair
Affinity DataIC50: 69nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Inhibition of human kidney type glutaminase (124 to 669 residues) using L-[3H]-glutamine as substrate after 45 mins by topcount methodMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Inhibition of human kidney type glutaminase (124 to 669 residues) using L-[3H]-glutamine as substrate after 45 mins by topcount methodMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Inhibition of human kidney type glutaminase (124 to 669 residues) using L-[3H]-glutamine as substrate after 45 mins by topcount methodMore data for this Ligand-Target Pair
Affinity DataIC50: 82nMpH: 7.4 T: 2°CAssay Description:GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ...More data for this Ligand-Target Pair
Affinity DataIC50: 90nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 90nMAssay Description:Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]GMore data for this Ligand-Target Pair
Affinity DataIC50: 90nMpH: 7.4 T: 2°CAssay Description:GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ...More data for this Ligand-Target Pair
Affinity DataIC50: 93nMAssay Description:Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]GMore data for this Ligand-Target Pair
Affinity DataIC50: 94nMAssay Description:Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]GMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of human kidney type glutaminase (124 to 669 residues) using L-[3H]-glutamine as substrate after 45 mins by topcount methodMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMpH: 7.4 T: 2°CAssay Description:GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibition of human kidney type glutaminase (124 to 669 residues) using L-[3H]-glutamine as substrate after 45 mins by topcount methodMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibition of human kidney type glutaminase (124 to 669 residues) using L-[3H]-glutamine as substrate after 45 mins by topcount methodMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibition of human kidney type glutaminase (124 to 669 residues) using L-[3H]-glutamine as substrate after 45 mins by topcount methodMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Inhibition of human kidney type glutaminase (124 to 669 residues) using L-[3H]-glutamine as substrate after 45 mins by topcount methodMore data for this Ligand-Target Pair
Affinity DataIC50: 140nMAssay Description:Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]GMore data for this Ligand-Target Pair
Affinity DataIC50: 140nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 170nMAssay Description:Inhibition of human kidney type glutaminase (124 to 669 residues) using L-[3H]-glutamine as substrate after 45 mins by topcount methodMore data for this Ligand-Target Pair
Affinity DataIC50: 190nMpH: 7.4 T: 2°CAssay Description:GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibition of human kidney type glutaminase (124 to 669 residues) using L-[3H]-glutamine as substrate after 45 mins by topcount methodMore data for this Ligand-Target Pair
Affinity DataIC50: 220nMpH: 7.4 T: 2°CAssay Description:GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ...More data for this Ligand-Target Pair
Affinity DataIC50: 230nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 340nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 380nMAssay Description:Inhibition of human kidney type glutaminase (124 to 669 residues) using L-[3H]-glutamine as substrate after 45 mins by topcount methodMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)