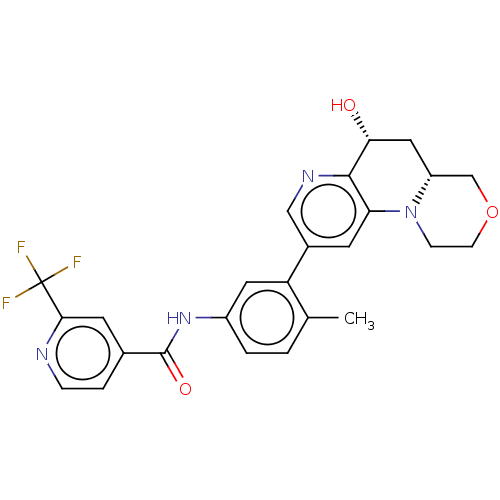
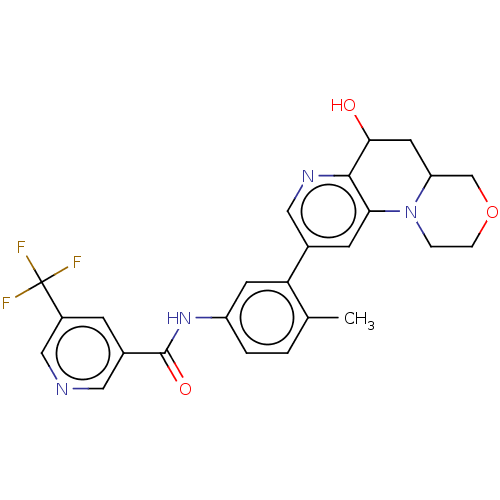
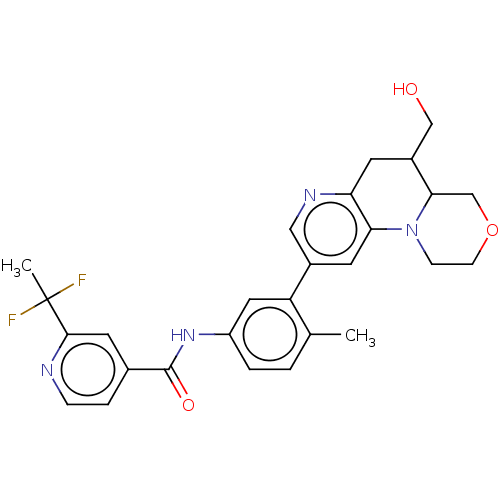
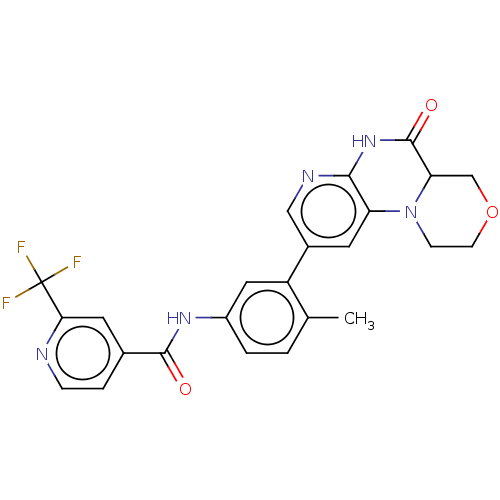
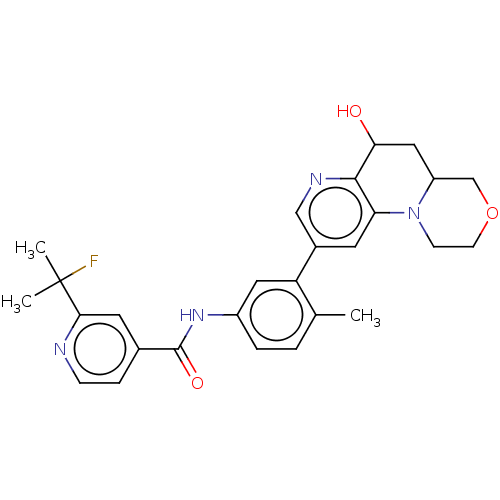
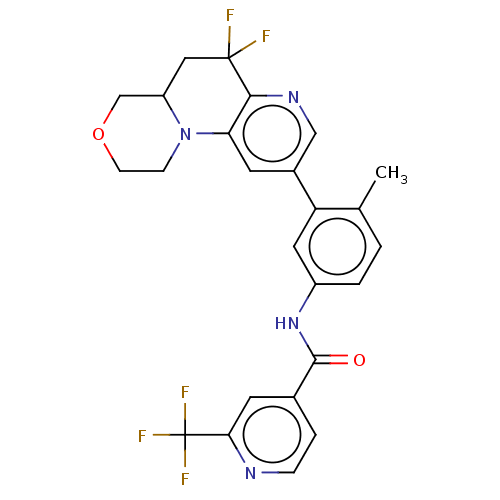
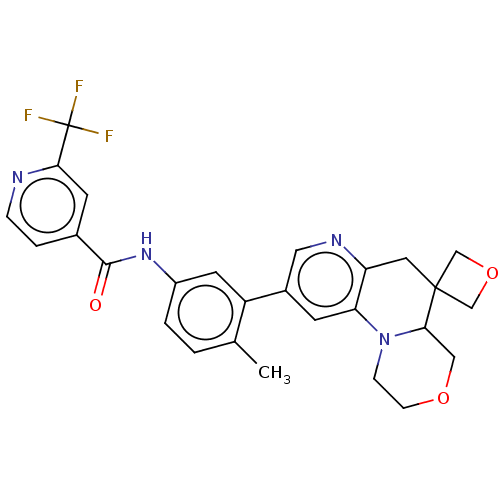
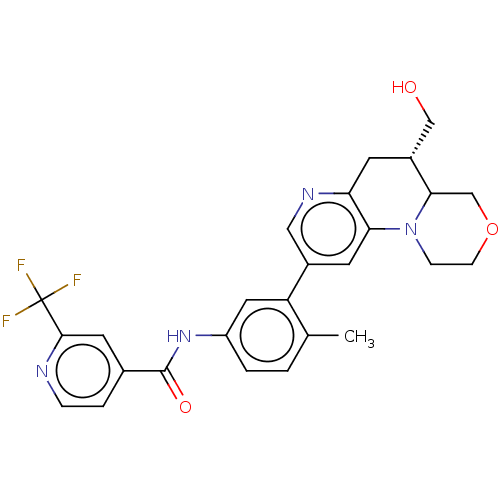
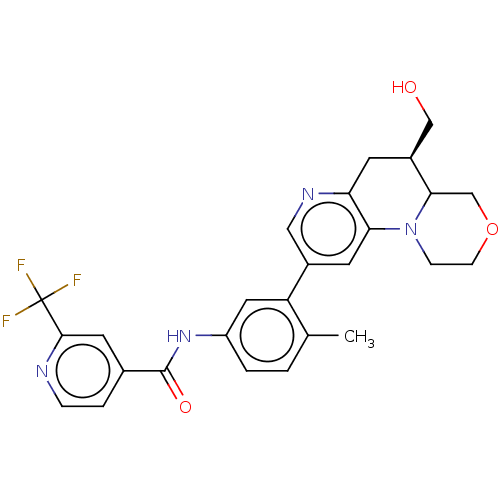
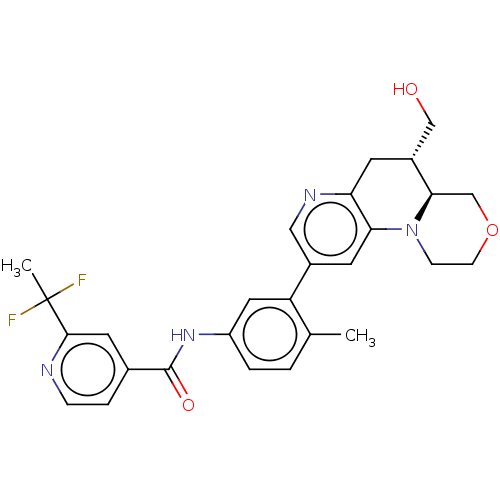
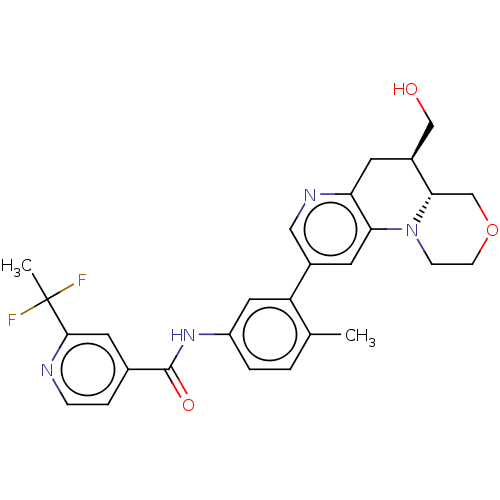
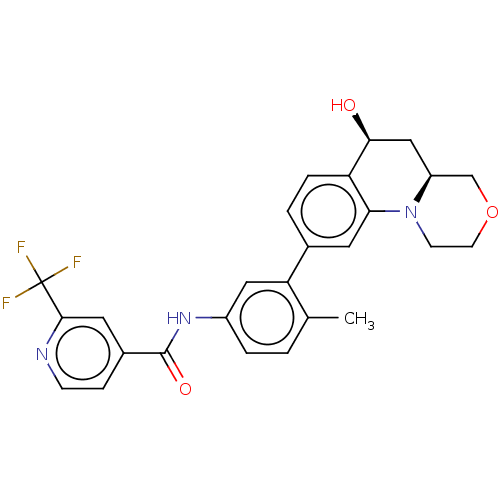
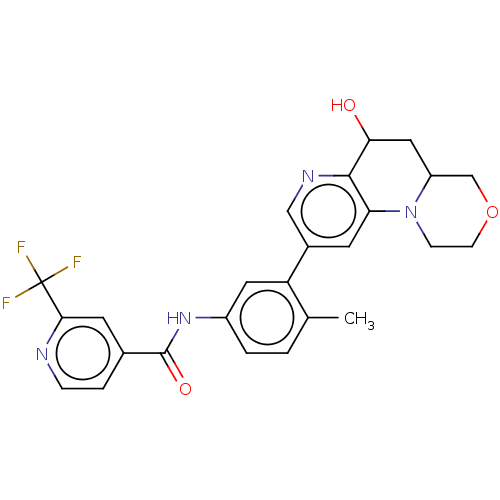
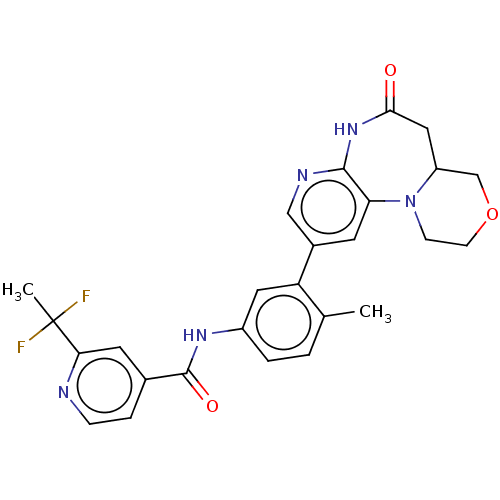
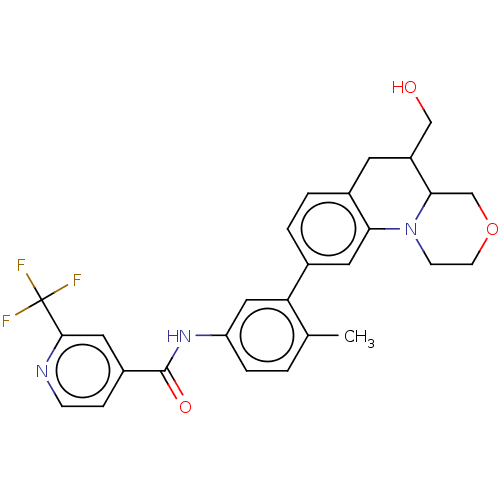
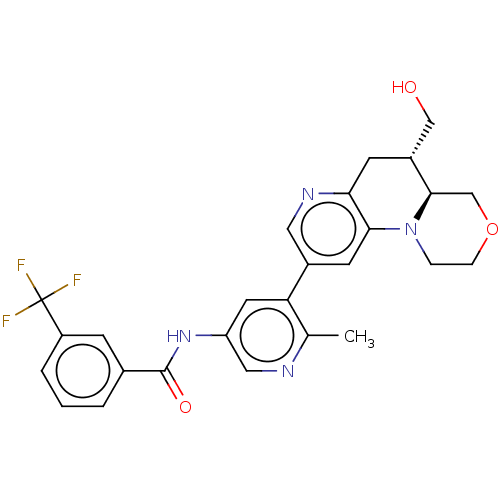
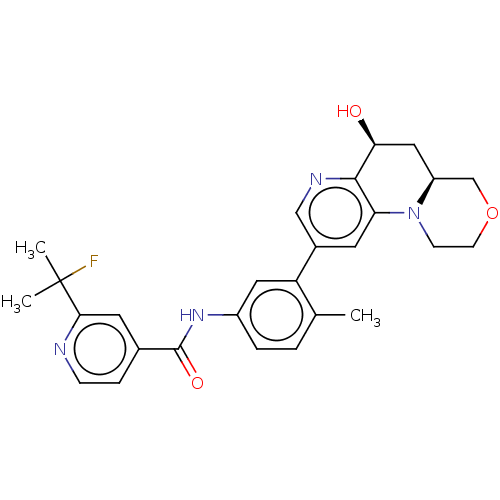
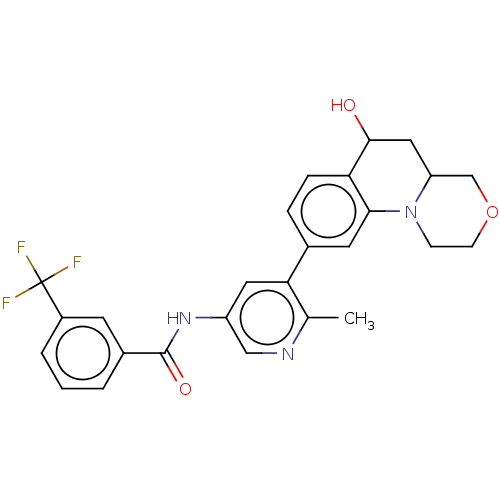
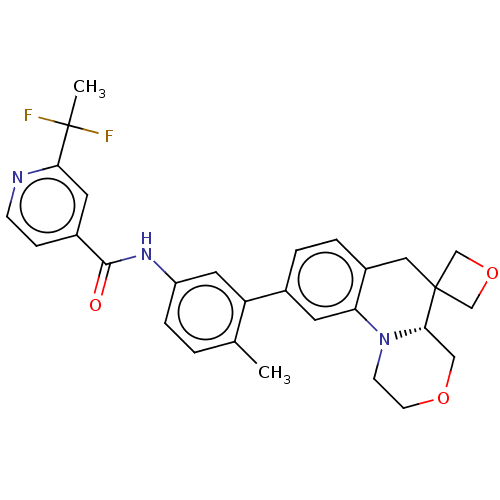
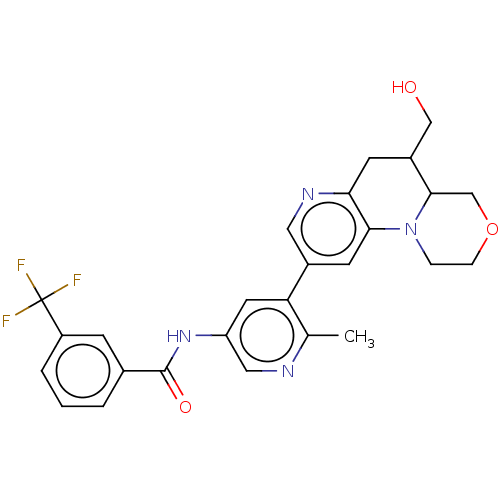
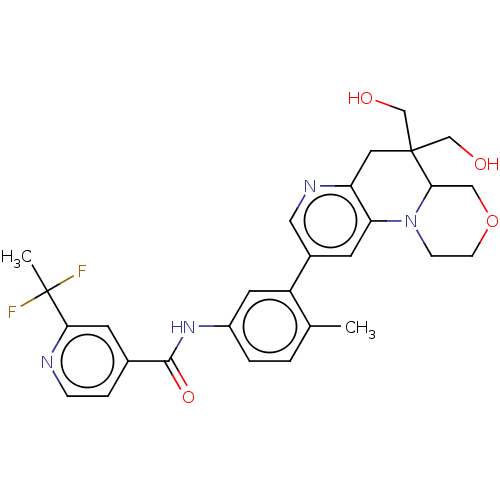
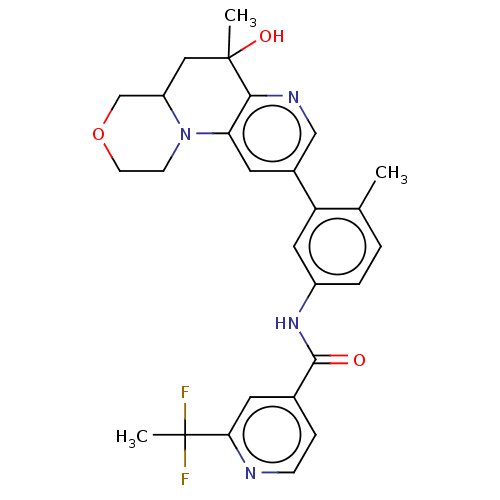
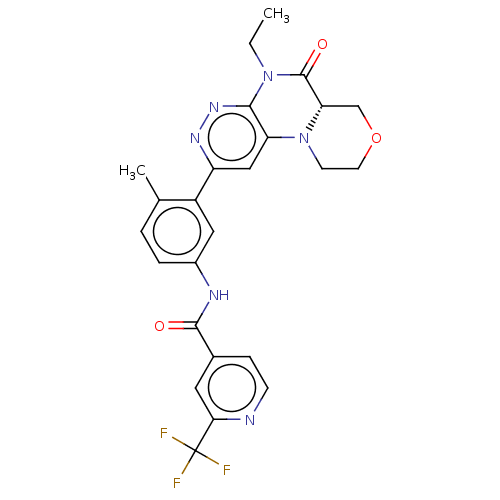
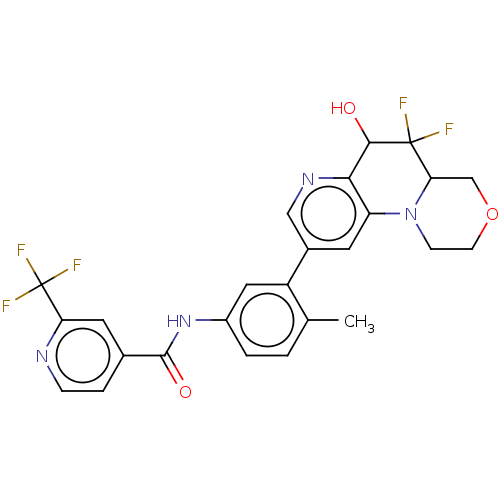
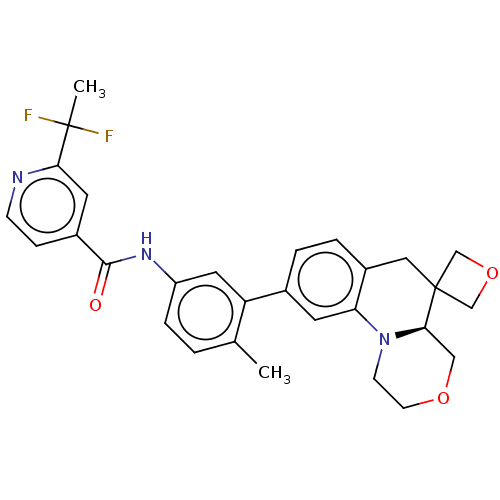
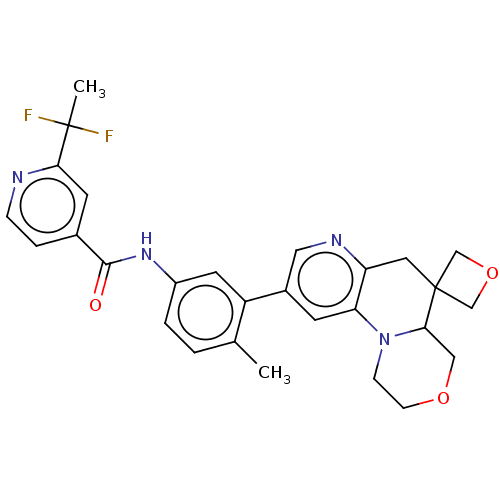
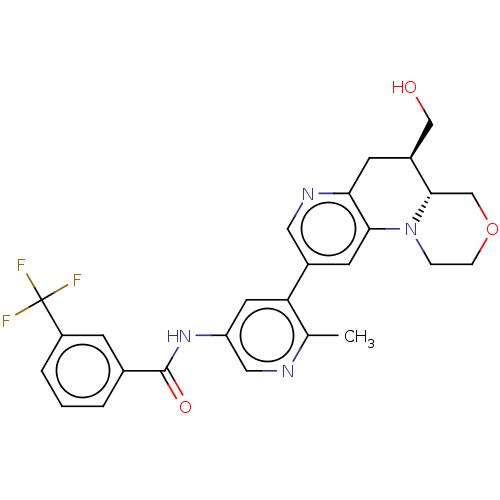
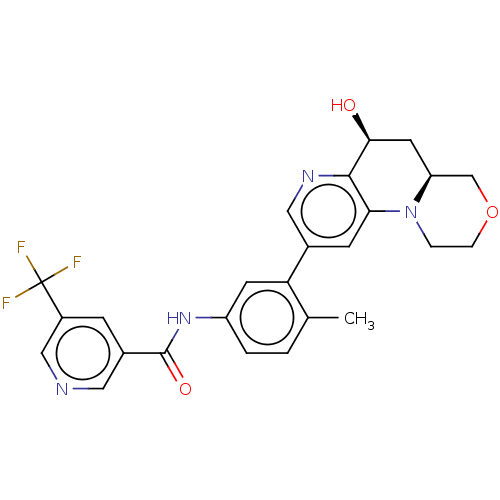
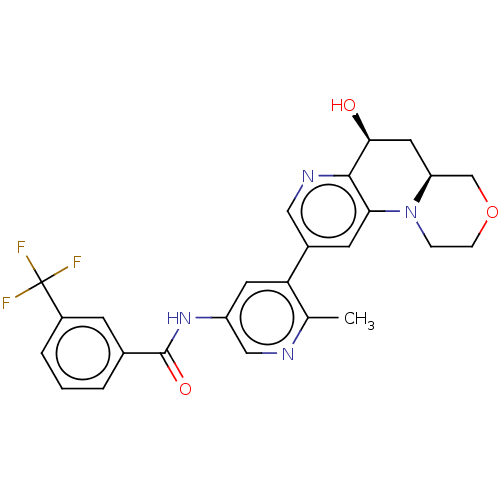
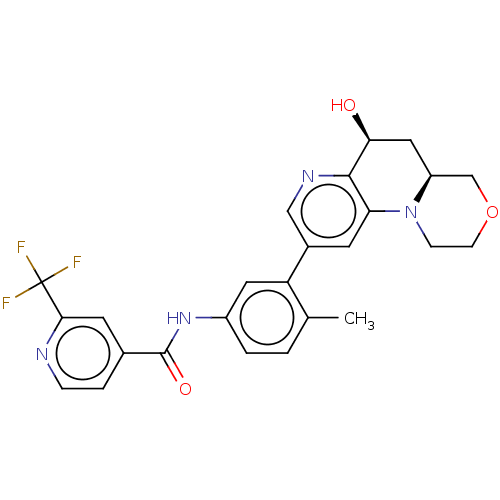
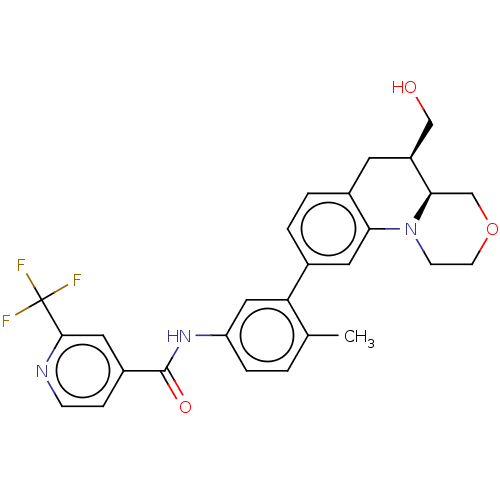
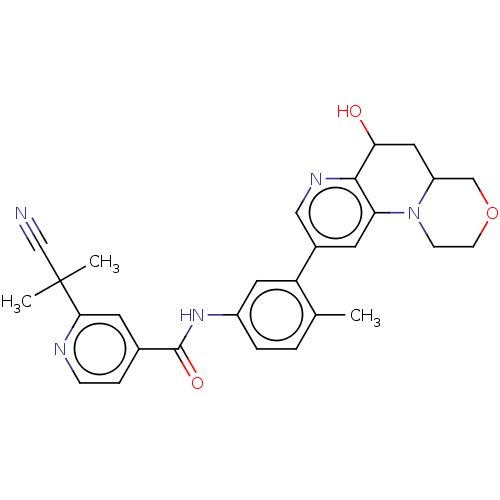
Report error Found 1923 Enz. Inhib. hit(s) with Target = 'RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E]'
Affinity DataIC50: 0.00100nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: <0.00200nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00200nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00200nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: <0.00200nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: <0.00200nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00200nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00300nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00400nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00500nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00500nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00500nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00500nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00500nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00500nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00500nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00600nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00600nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00600nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00600nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00700nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00700nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00800nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00800nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00800nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00800nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00900nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00900nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00900nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00900nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00900nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00900nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0100nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0100nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0100nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0100nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0100nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0110nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0110nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0110nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0110nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0110nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0110nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0120nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0120nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0120nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0130nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0130nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0130nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0130nMAssay Description:The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ...More data for this Ligand-Target Pair