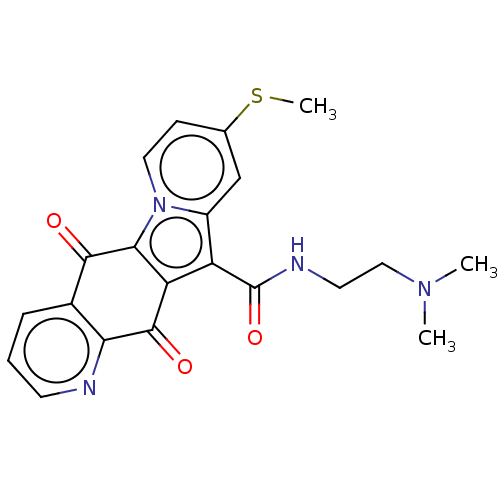
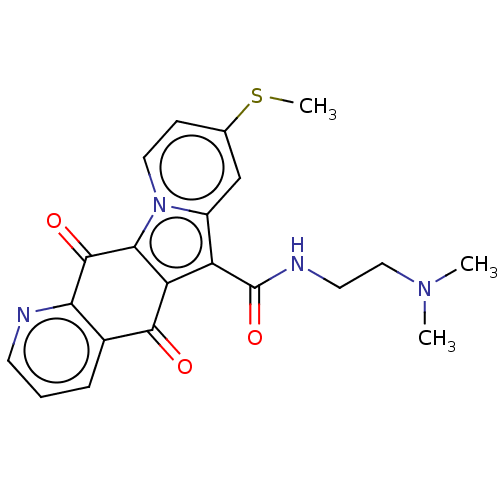
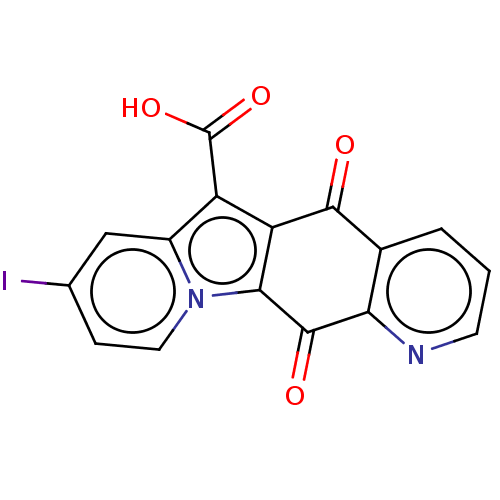
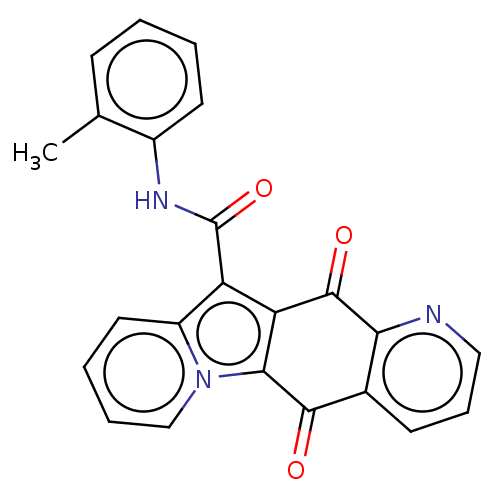
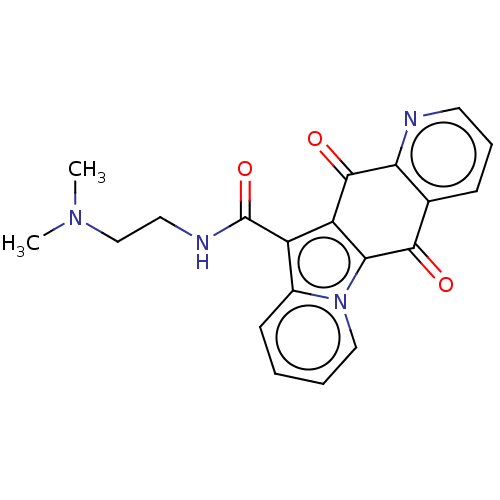
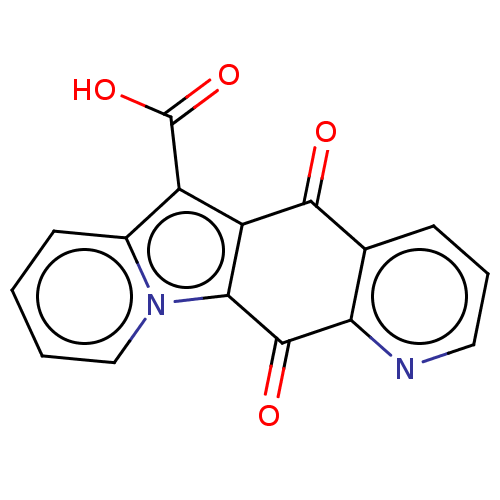
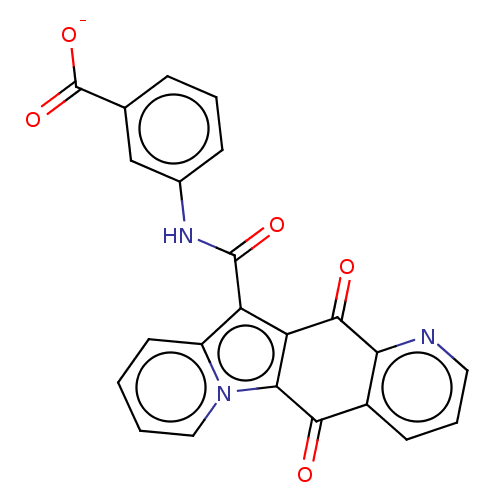
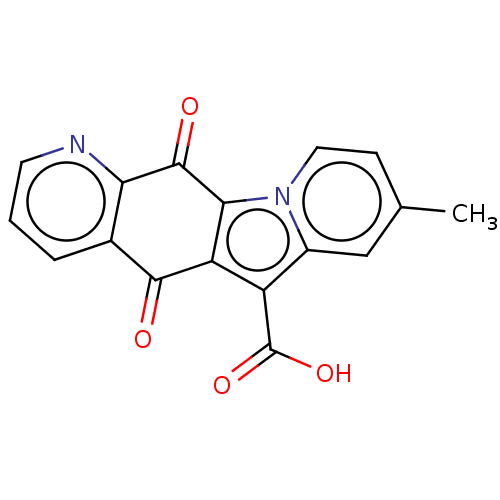
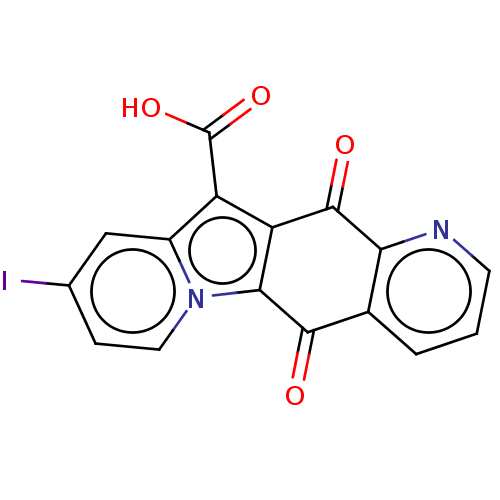
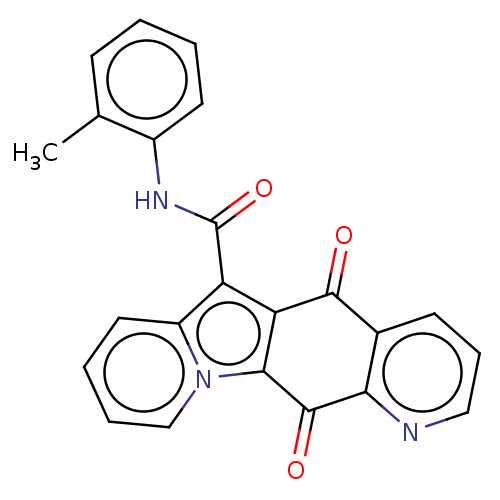
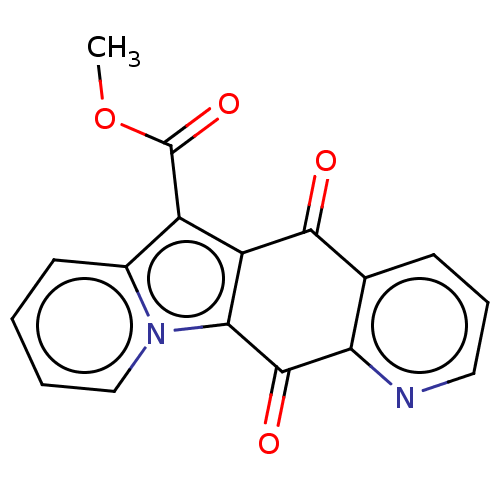
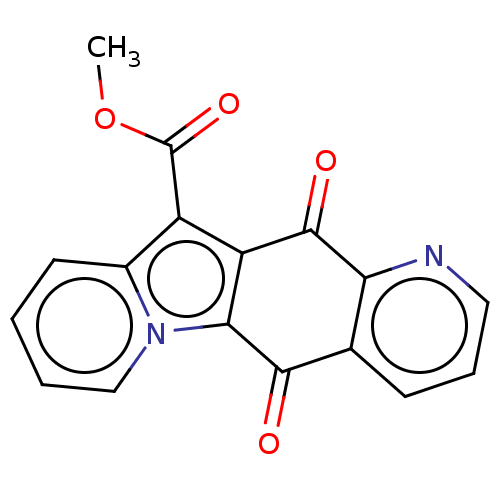
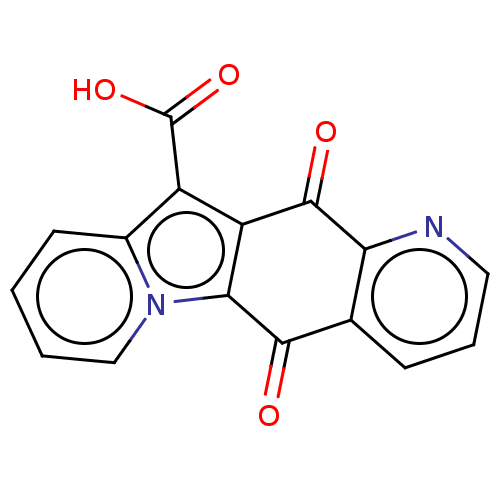
Affinity DataIC50: 7nMAssay Description:Inhibition of IFN-gamma-stimulated IDO1 in human HeLa cells after 24 hrs by Ehrlich's reagent based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 73nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 87nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 117nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 119nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 127nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 134nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 148nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 156nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 188nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 288nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 299nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 332nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 361nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 372nMAssay Description:Inhibition of IFN-gamma-stimulated IDO1 in human HeLa cells after 24 hrs by Ehrlich's reagent based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 390nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 394nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 420nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 427nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 438nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 448nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 714nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 738nMAssay Description:Inhibition of IFN-gamma-stimulated IDO1 in human HeLa cells after 24 hrs by Ehrlich's reagent based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 977nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.08E+3nMAssay Description:Inhibition of IFN-gamma-stimulated IDO1 in human HeLa cells after 24 hrs by Ehrlich's reagent based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.19E+3nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in N'-formlylkynurenine formation using D-Tryptophan as substrate in presence of aspartate ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of recombinant TDO (unknown origin) assessed as reduction in N-formylkynurenine formation using L-Tryptophan as substrate after 75 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 1.24E+3nMAssay Description:Inhibition of IFN-gamma-stimulated IDO1 in human HeLa cells after 24 hrs by Ehrlich's reagent based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.66E+3nMAssay Description:Inhibition of IFN-gamma-stimulated IDO1 in human HeLa cells after 24 hrs by Ehrlich's reagent based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.35E+3nMAssay Description:Inhibition of recombinant TDO (unknown origin) assessed as reduction in N-formylkynurenine formation using L-Tryptophan as substrate after 75 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 7.15E+3nMAssay Description:Inhibition of recombinant TDO (unknown origin) assessed as reduction in N-formylkynurenine formation using L-Tryptophan as substrate after 75 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 7.30E+3nMAssay Description:Inhibition of recombinant TDO (unknown origin) assessed as reduction in N-formylkynurenine formation using L-Tryptophan as substrate after 75 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 4.16E+4nMAssay Description:Inhibition of recombinant TDO (unknown origin) assessed as reduction in N-formylkynurenine formation using L-Tryptophan as substrate after 75 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of recombinant TDO (unknown origin) assessed as reduction in N-formylkynurenine formation using L-Tryptophan as substrate after 75 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of recombinant TDO (unknown origin) assessed as reduction in N-formylkynurenine formation using L-Tryptophan as substrate after 75 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of recombinant TDO (unknown origin) assessed as reduction in N-formylkynurenine formation using L-Tryptophan as substrate after 75 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of recombinant TDO (unknown origin) assessed as reduction in N-formylkynurenine formation using L-Tryptophan as substrate after 75 mins by...More data for this Ligand-Target Pair