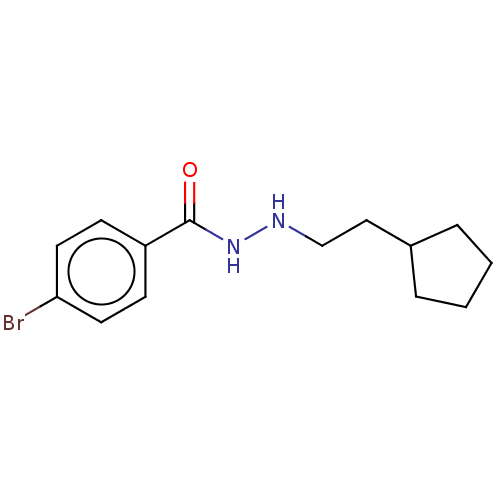
BDBM163632 SR-3297::US10807944, Compound RLS2-219::US11731934, Compound RLS2-219
SMILES Brc1ccc(cc1)C(=O)NNCCC1CCCC1
InChI Key InChIKey=ZLEUBTRYAAACNU-UHFFFAOYSA-N
Data 12 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 163632
Found 12 hits for monomerid = 163632
TargetHistone deacetylase 1/Nuclear receptor corepressor 2 [395-498](Homo sapiens (Human))
University of Florida College of Medicine
University of Florida College of Medicine
Affinity DataIC50: 7.40E+3nMAssay Description:Purified HDAC1, HDAC2, and HDAC3 (in complex with the deacetylase activation domain of the human NCOR2 (amino acids 395¿498)) were obtained from BPS ...More data for this Ligand-Target Pair
TargetHistone deacetylase 2/Nuclear receptor corepressor 2 [395-498](Homo sapiens (Human))
University of Florida College of Medicine
University of Florida College of Medicine
Affinity DataIC50: 7.65E+3nMAssay Description:Purified HDAC1, HDAC2, and HDAC3 (in complex with the deacetylase activation domain of the human NCOR2 (amino acids 395¿498)) were obtained from BPS ...More data for this Ligand-Target Pair
TargetHistone deacetylase 3/Nuclear receptor corepressor 2 [395-498](Homo sapiens (Human))
University of Florida College of Medicine
University of Florida College of Medicine
Affinity DataIC50: 1.08E+3nMAssay Description:Purified HDAC1, HDAC2, and HDAC3 (in complex with the deacetylase activation domain of the human NCOR2 (amino acids 395¿498)) were obtained from BPS ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.40E+3nMAssay Description:These SAR data indicate that a tripartite structure of this scaffold with a central —C(O)—NH—NH— unit flanked by a phenyl group and a short aliphatic...More data for this Ligand-Target Pair
Affinity DataIC50: 7.65E+3nMAssay Description:These SAR data indicate that a tripartite structure of this scaffold with a central —C(O)—NH—NH— unit flanked by a phenyl group and a short aliphatic...More data for this Ligand-Target Pair
Affinity DataIC50: 7.40E+3nMAssay Description:Inhibition of HDAC1 (unknown origin) using acetylated 7-amino-4-methylcoumarin (AMC) as peptide substrate measured after 20 mins by fluorescence base...More data for this Ligand-Target Pair
Affinity DataIC50: 7.65E+3nMAssay Description:Inhibition of HDAC2 (unknown origin) using acetylated 7-amino-4-methylcoumarin (AMC) as peptide substrate measured after 20 mins by fluorescence base...More data for this Ligand-Target Pair
Affinity DataIC50: 1.08E+3nMAssay Description:Inhibition of HDAC3 (unknown origin) using acetylated 7-amino-4-methylcoumarin (AMC) as peptide substrate measured after 20 mins by fluorescence base...More data for this Ligand-Target Pair
Affinity DataIC50: 7.40E+3nMAssay Description:These SAR data indicate that a tripartite structure of this scaffold with a central C(O) NH NH unit flanked by a phenyl group and a short aliphatic c...More data for this Ligand-Target Pair
Affinity DataIC50: 1.08E+3nMAssay Description:These SAR data indicate that a tripartite structure of this scaffold with a central —C(O)—NH—NH— unit flanked by a phenyl group and a short aliphatic...More data for this Ligand-Target Pair