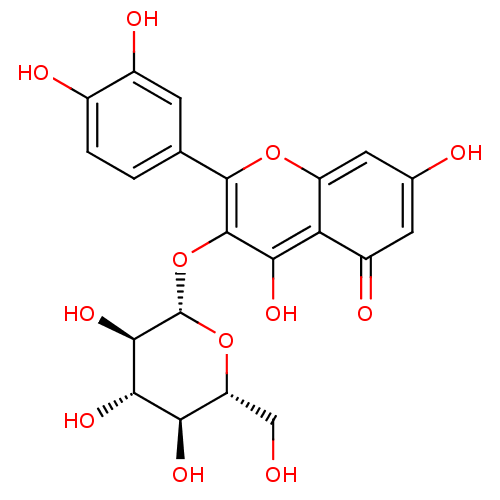
BDBM50241354 2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-(3,4,5-trihydroxy-6-hydroxymethyl-tetrahydro-pyran-2-yloxy)-chromen-4-one::2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-((2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)-tetrahydro-2H-pyran-2-yloxy)-4H-chromen-4-one::2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)-tetrahydro-2H-pyran-2-yloxy)-4H-chromen-4-one::2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yloxy)-4H-chromen-4-one::3,3',4',5,7-pentahydroxyflavone-3-beta-O-glucoside::3-glucoside isoquercitrin::CHEMBL250450::Hyperin::Hyperoside::NSC-407304::Quercetin 3-O-beta-D-glucoside::Quercetin-3-O-beta-D-galactopyranoside::Quercetin-3-glucoside::cid_5280804::cid_5378597::hirsutrin::hydroside::isoquercetin::isoquercetrin::quercetin -3-O-beta-D-galactopyranoside::quercetin 3-O-beta-D-galactopyranoside::quercetin 3-O-beta-D-glucopyranoside::quercetin 3-O-galactoside::quercetin 3-O-glucopyranoside::quercetin 3-O-glucoside::quercetin-3-galactoside
SMILES OC[C@H]1O[C@@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)[C@H](O)[C@@H](O)[C@@H]1O
InChI Key InChIKey=DPFYPHSPTUZJJT-QSOFNFLRSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 24 hits for monomerid = 50241354
Found 24 hits for monomerid = 50241354
Eberhard Karls University of Tuebingen
Chungnam National University
Curated by ChEMBL
Freie Universitaet Berlin
Curated by ChEMBL
Freie Universitaet Berlin
Curated by ChEMBL
Chungbuk National University
Curated by ChEMBL
King'S College London
Curated by ChEMBL
Dongguk University-Seoul
Curated by ChEMBL
Korean Institute Of Oriental Medicine (Kiom)
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Eberhard Karls University of Tuebingen
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Kyung Hee University
Curated by ChEMBL
Sanford-Burnham Center For Chemical Genomics
Curated by PubChem BioAssay