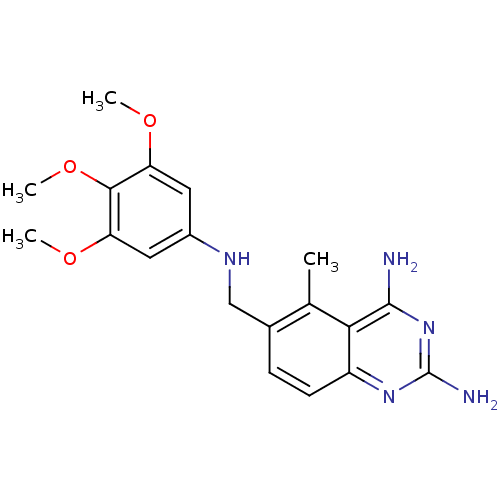
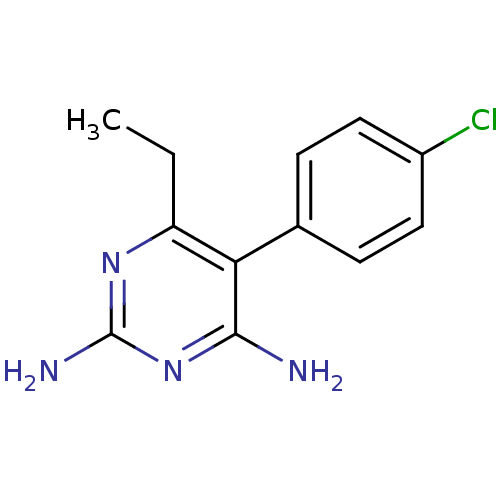
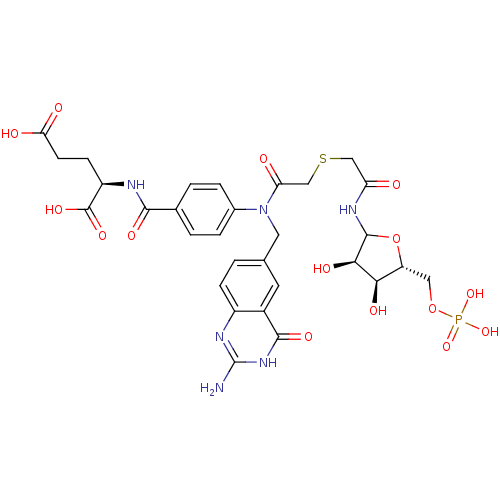
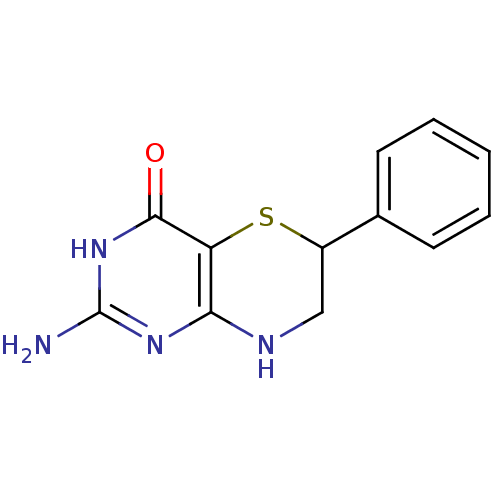
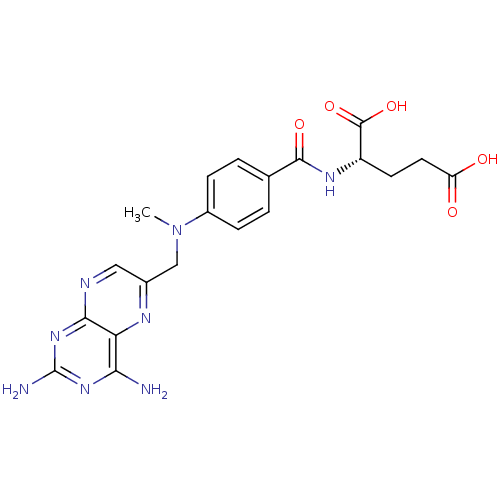
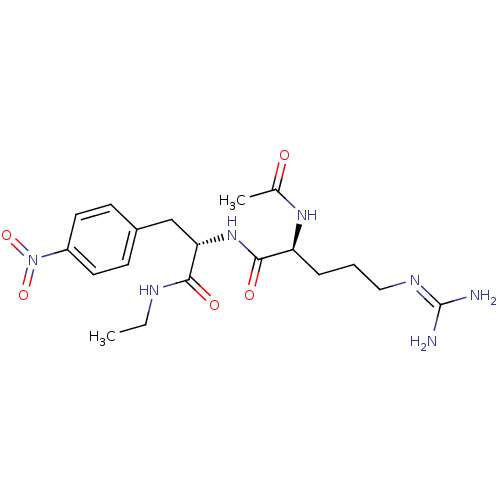
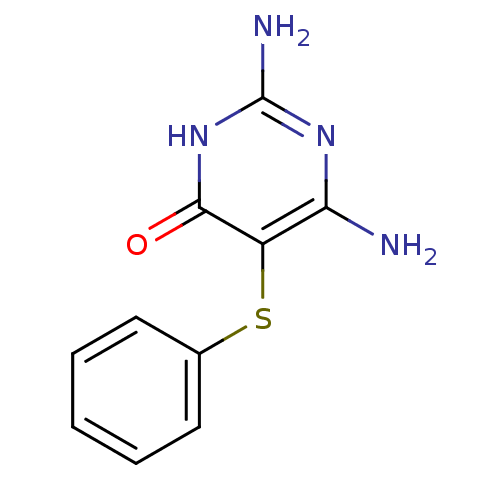
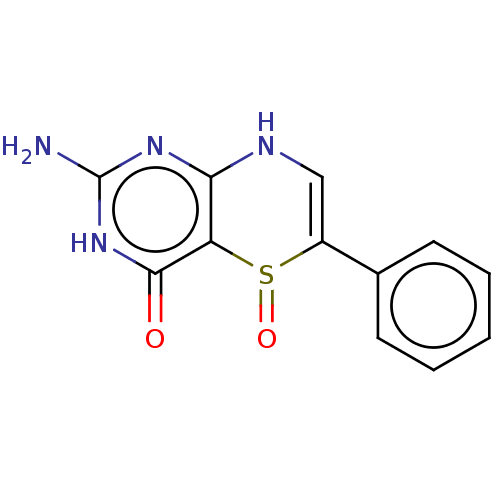
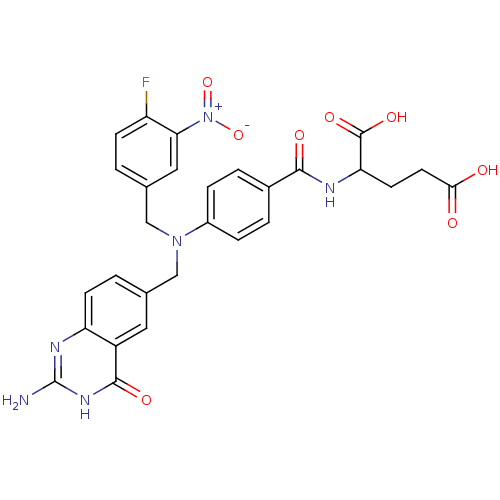
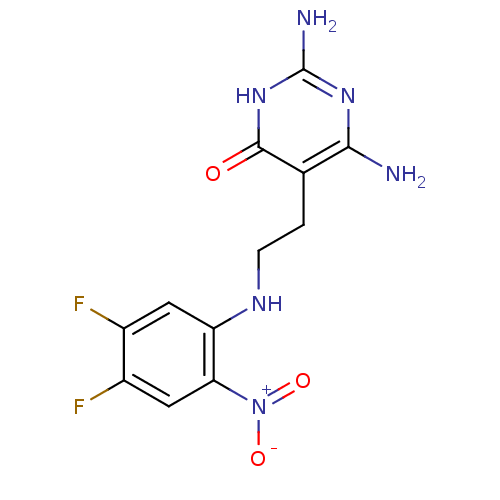
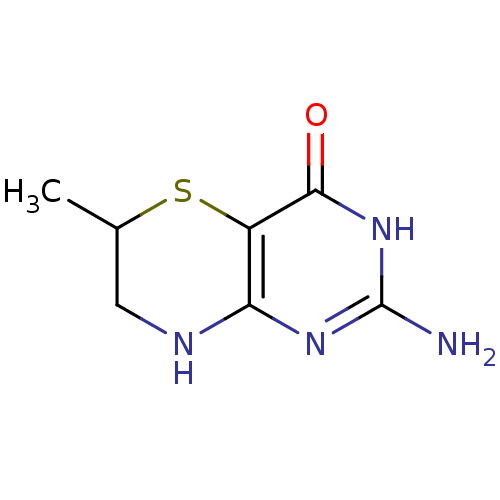
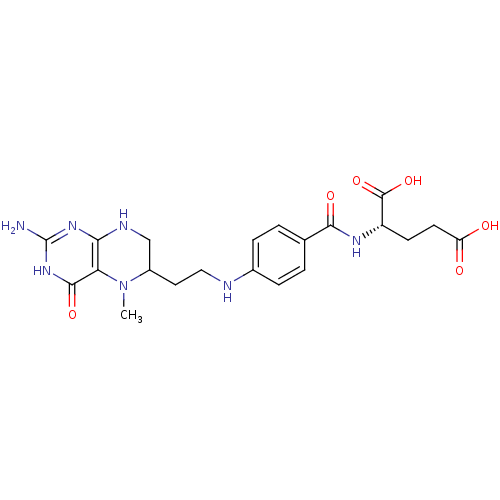
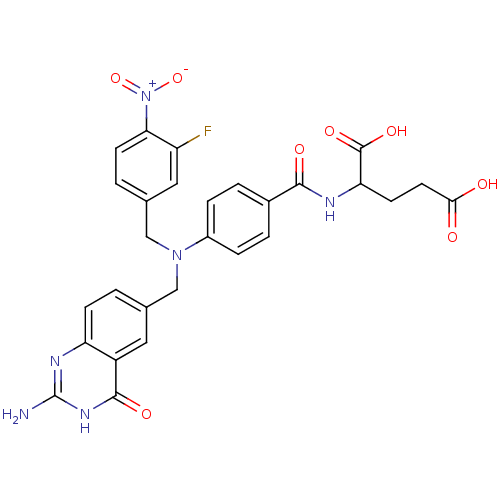
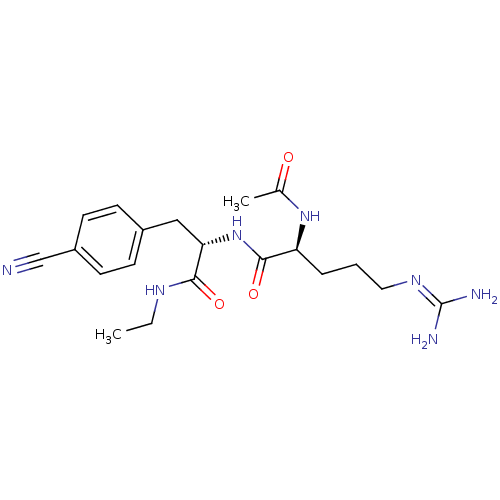
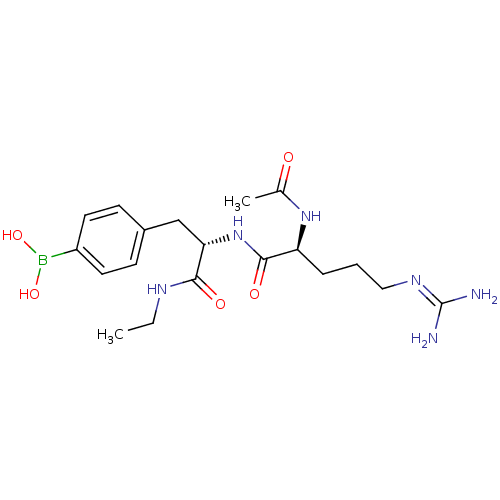
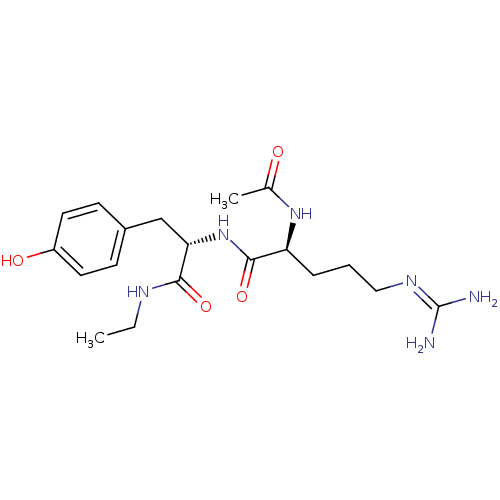
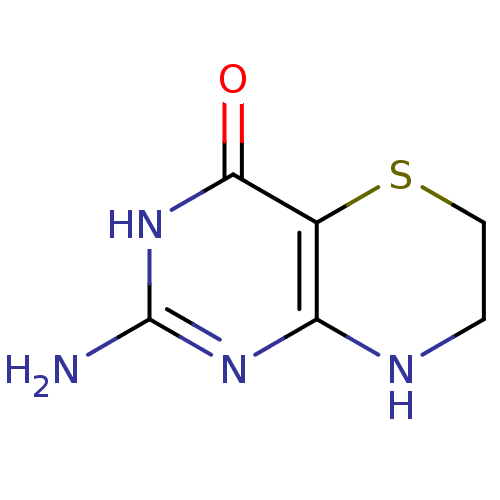
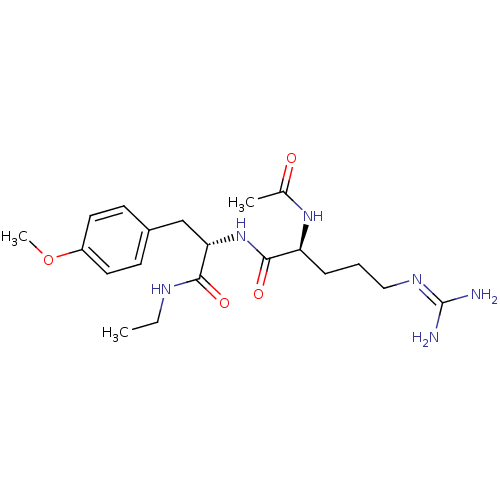
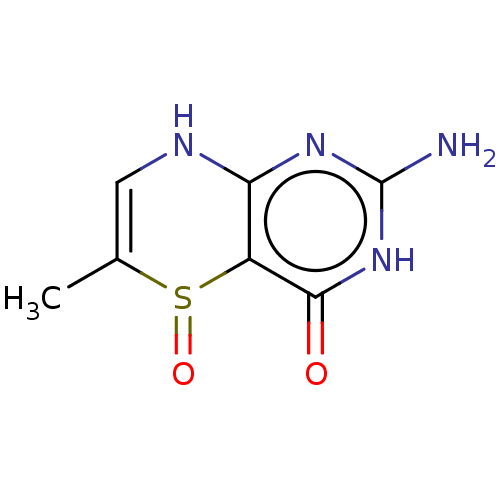
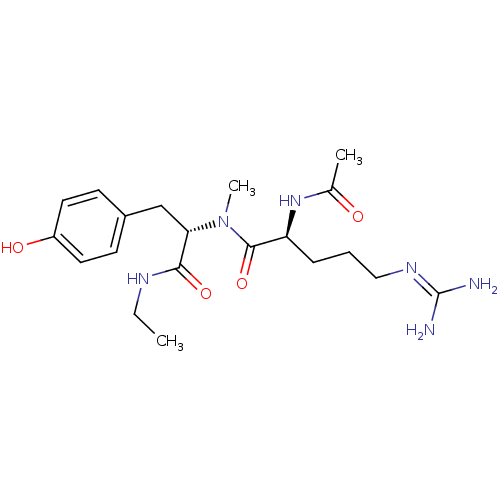
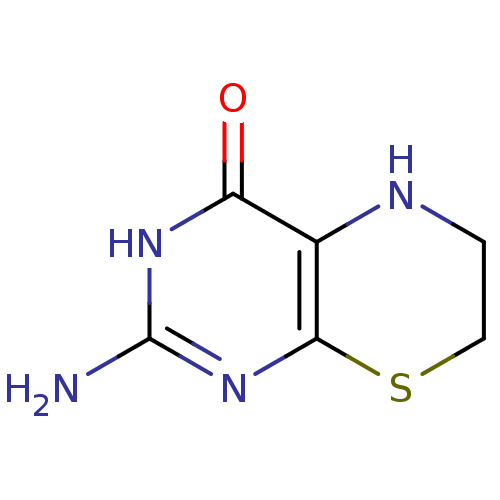
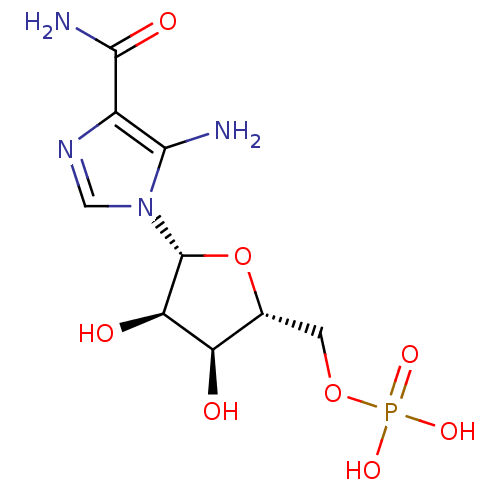
Affinity DataKi: 0.0180nMAssay Description:Inhibitory constant of the compound for murine Wild-type dihydrofolate reductase (DHFR)More data for this Ligand-Target Pair
Affinity DataKi: 0.0330nMAssay Description:Inhibitory constant of the compound for murine Wild-type dihydrofolate reductase (DHFR)More data for this Ligand-Target Pair
Affinity DataKi: 0.0420nMAssay Description:Inhibitory constant of the compound for murine Wild-type dihydrofolate reductase (DHFR)More data for this Ligand-Target Pair
Affinity DataKi: 0.0600nMAssay Description:Inhibitory constant of the compound for murine Wild-type dihydrofolate reductase (DHFR)More data for this Ligand-Target Pair
TargetBifunctional dihydrofolate reductase-thymidylate synthase(Plasmodium falciparum (isolate K1 / Thailand))
Pennsylvania State University
Curated by ChEMBL
Pennsylvania State University
Curated by ChEMBL
Affinity DataKi: 0.190nMAssay Description:Inhibitor constant of compound for plasmodium falciparum dihydrofolate reductaseMore data for this Ligand-Target Pair
TargetBifunctional dihydrofolate reductase-thymidylate synthase(Plasmodium falciparum (isolate K1 / Thailand))
Pennsylvania State University
Curated by ChEMBL
Pennsylvania State University
Curated by ChEMBL
Affinity DataKi: 0.190nMAssay Description:Thermodynamic dissociation constant of compound for mutant T46S E. coli dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.810nMAssay Description:Inhibitory constant of the compound for F31A/F34A murine dihydrofolate reductase (DHFR)More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Inhibitor constant of compound for mutant S108 N plasmodium falciparum i dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 5.20nMAssay Description:Dissociation rate constant of compound for mutant T46N Escherichia coli dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Dissociation rate constant of compound for mutant T46A Escherichia coli dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibitor constant of compound for mutant T46S E. coli dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 25.9nMAssay Description:Inhibitory constant of the compound for F31A/F34A murine dihydrofolate reductase (DHFR)More data for this Ligand-Target Pair
Affinity DataKi: 44nMAssay Description:Thermodynamic Dissociation Constant for compound-Tyr31-dihydrofolate reductase (DHFR) complex at pH 8More data for this Ligand-Target Pair
Affinity DataKi: 57nMAssay Description:Inhibition constant for Tyr31-dihydrofolate reductase (DHFR)-NADPH-Compound complexMore data for this Ligand-Target Pair
TargetTrifunctional purine biosynthetic protein adenosine-3(Mus musculus)
Pennsylvania State University
Curated by ChEMBL
Pennsylvania State University
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:Compound was tested for inhibition constant against GAR TFase in miceMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Tested for competitive binding inhibition against phenylalanine hydroxylase in rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 212nMAssay Description:Inhibitory constant of the compound for F31A/F34A murine dihydrofolate reductase (DHFR)More data for this Ligand-Target Pair
Affinity DataKi: 330nMAssay Description:Thermodynamic Dissociation Constant for compound-Tyr31-dihydrofolate reductase (DHFR) complex at pH 9.5More data for this Ligand-Target Pair
Affinity DataKi: 333nMAssay Description:Inhibitory constant of the compound for F31A/F34A murine dihydrofolate reductase (DHFR)More data for this Ligand-Target Pair
Affinity DataKi: 685nM ΔG°: -35.2kJ/moleT: 2°CAssay Description:Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC).More data for this Ligand-Target Pair
TargetLeucine--tRNA ligase, cytoplasmic(Saccharomyces cerevisiae S288c)
Anacor Pharmaceuticals
Curated by ChEMBL
Anacor Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 1.85E+3nMAssay Description:Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase after 20 minsMore data for this Ligand-Target Pair
Affinity DataKi: 2.40E+3nM ΔG°: -32.1kJ/molepH: 5.5 T: 2°CAssay Description:Thermodynamic Dissociation Constant for compound-Val31-dihydrofolate reductase (DHFR) complex at pH 7More data for this Ligand-Target Pair
Affinity DataKi: 2.50E+3nM ΔG°: -32.0kJ/moleT: 2°CAssay Description:Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC).More data for this Ligand-Target Pair
Affinity DataKi: 4.70E+3nMAssay Description:Tested for competitive binding inhibition against phenylalanine hydroxylase in rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 7.00E+3nMAssay Description:Tested for competitive binding inhibition against phenylalanine hydroxylase in rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 9.10E+3nMAssay Description:Dissociation constant at(Koff) Tyr-31 of dihydrofolate reductase (DHFR)More data for this Ligand-Target Pair
Affinity DataKi: 1.70E+4nMAssay Description:In vitro inhibitory activity against Aminoimidazole carboxamide ribonucleotide transformylaseMore data for this Ligand-Target Pair
TargetLeucine--tRNA ligase, cytoplasmic(Saccharomyces cerevisiae S288c)
Anacor Pharmaceuticals
Curated by ChEMBL
Anacor Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 3.14E+4nMAssay Description:Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase after 2 minsMore data for this Ligand-Target Pair
TargetTrifunctional purine biosynthetic protein adenosine-3(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 3.50E+4nMAssay Description:In vitro inhibitory activity against Glycinamide ribonucleotide transformylase (GAR Tfase) after 3 min at 250 uMMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+4nMAssay Description:Tested for competitive binding inhibition against phenylalanine hydroxylase in rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 4.10E+4nMAssay Description:Compound was tested for in vitro inhibition of 5,10-Methylene Tetrahydrofolate Cyclohydrolase ,competitive against (+)-L-5,10-methenyltetrahydrofolat...More data for this Ligand-Target Pair
TargetTrifunctional purine biosynthetic protein adenosine-3(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 4.50E+4nMAssay Description:In vitro inhibitory activity against Glycinamide ribonucleotide transformylase (GAR Tfase)More data for this Ligand-Target Pair
Affinity DataKi: 5.20E+4nMAssay Description:In vitro inhibitory activity against Aminoimidazole carboxamide ribonucleotide transformylaseMore data for this Ligand-Target Pair
Affinity DataKi: 5.60E+4nM ΔG°: -24.3kJ/moleT: 2°CAssay Description:Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC).More data for this Ligand-Target Pair
Affinity DataKi: 5.90E+4nM ΔG°: -24.1kJ/moleT: 2°CAssay Description:Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC).More data for this Ligand-Target Pair
Affinity DataKi: 8.40E+4nM ΔG°: -23.3kJ/moleT: 2°CAssay Description:Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC).More data for this Ligand-Target Pair
Affinity DataKi: 8.50E+4nMAssay Description:Tested for competitive binding inhibition against phenylalanine hydroxylase in rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 8.70E+4nM ΔG°: -23.2kJ/moleT: 2°CAssay Description:Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC).More data for this Ligand-Target Pair
TargetTrifunctional purine biosynthetic protein adenosine-3(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:In vitro inhibitory activity against Glycinamide ribonucleotide transformylase (GAR Tfase)More data for this Ligand-Target Pair
Affinity DataKi: 1.25E+5nMAssay Description:Tested for competitive binding inhibition against phenylalanine hydroxylase in rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 1.35E+5nM ΔG°: -22.1kJ/moleT: 2°CAssay Description:Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC).More data for this Ligand-Target Pair
Affinity DataKi: 1.45E+5nM ΔG°: -21.9kJ/moleT: 2°CAssay Description:Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC).More data for this Ligand-Target Pair
Affinity DataKi: 1.70E+5nMAssay Description:Thermodynamic Dissociation Constant for compound-Phe31-dihydrofolate reductase (DHFR) complex at pH 8.5More data for this Ligand-Target Pair
Affinity DataKi: 3.39E+5nM ΔG°: -19.8kJ/moleT: 2°CAssay Description:Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC).More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+5nMAssay Description:Tested for competitive binding inhibition against phenylalanine hydroxylase in rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 5.00E+5nMAssay Description:Tested for competitive binding inhibition against phenylalanine hydroxylase in rat liverMore data for this Ligand-Target Pair
Affinity DataIC50: 125nMAssay Description:Inhibitory activity against AICAR formyltransferaseMore data for this Ligand-Target Pair
TargetLeucine--tRNA ligase, cytoplasmic(Saccharomyces cerevisiae S288c)
Anacor Pharmaceuticals
Curated by ChEMBL
Anacor Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase assessed as tRNA amino-acylationMore data for this Ligand-Target Pair
Affinity DataIC50: 6.40E+3nMAssay Description:Inhibitory activity against AICAR formyltransferaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibitory activity against AICAR formyltransferaseMore data for this Ligand-Target Pair

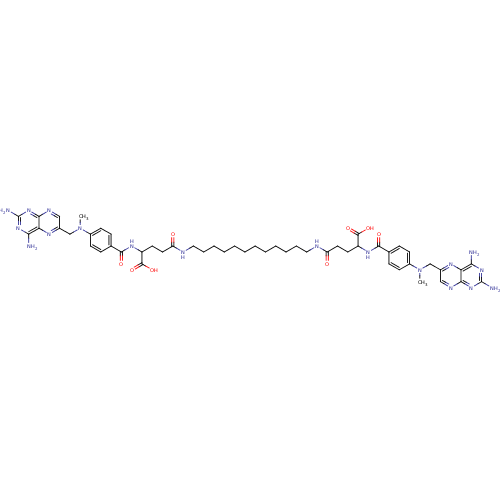

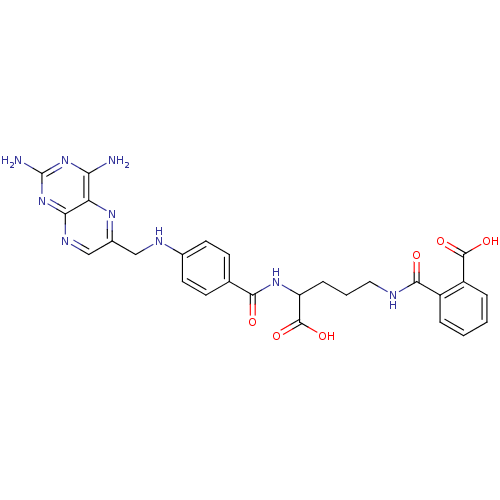
 3D Structure (crystal)
3D Structure (crystal)