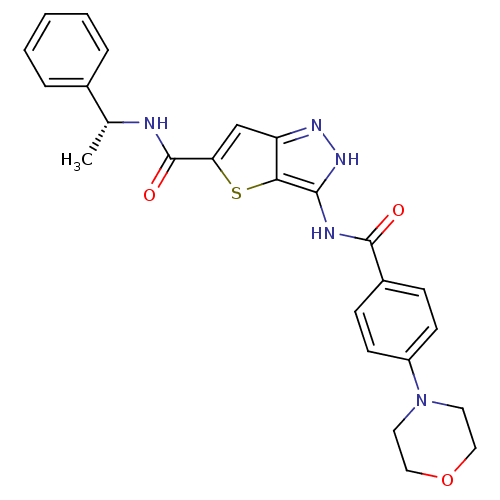
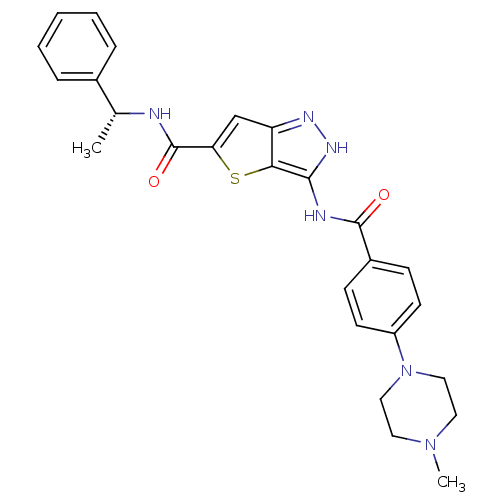
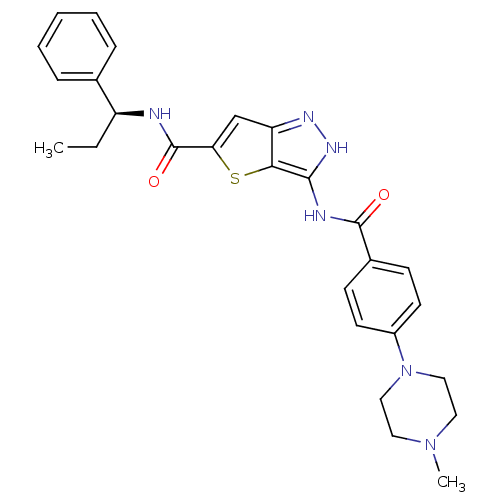
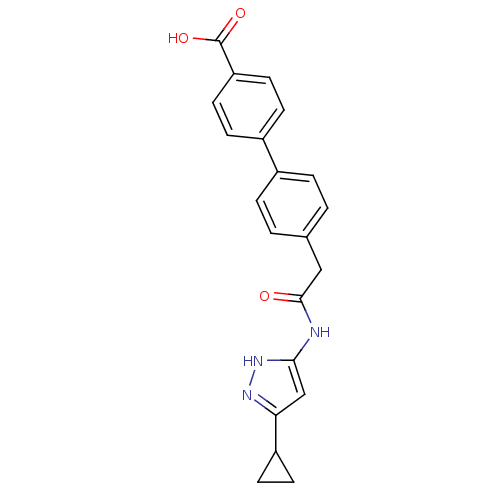
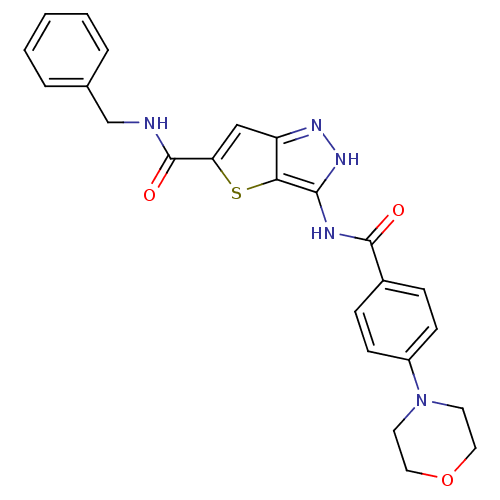
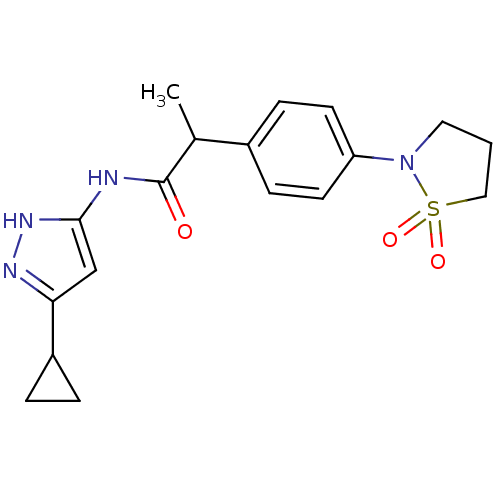
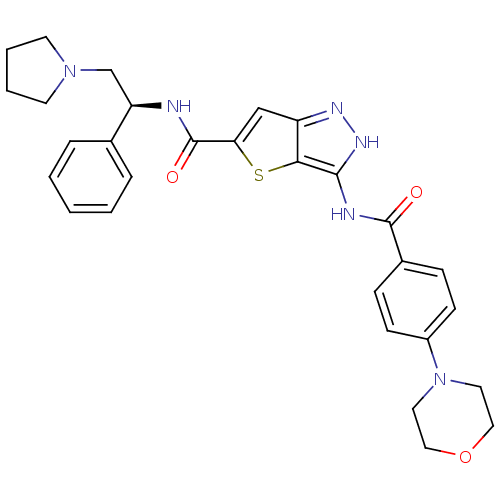
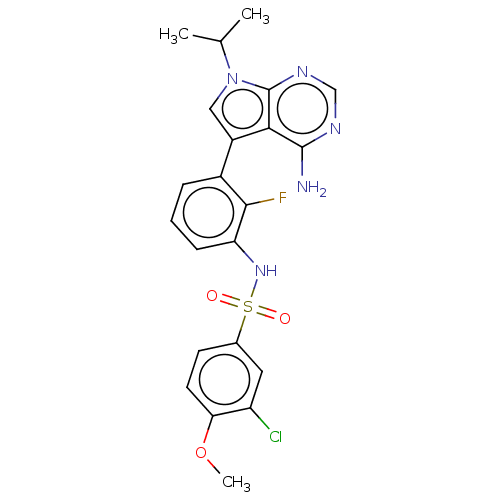
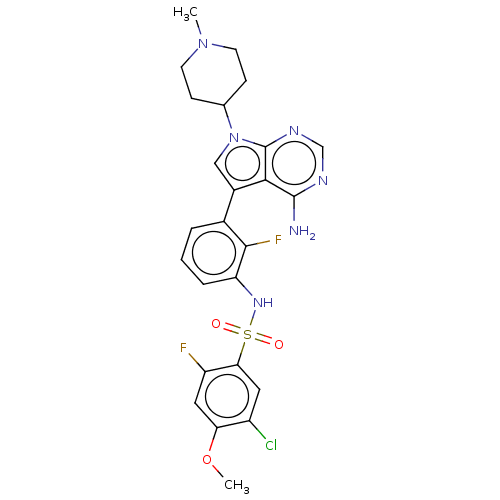
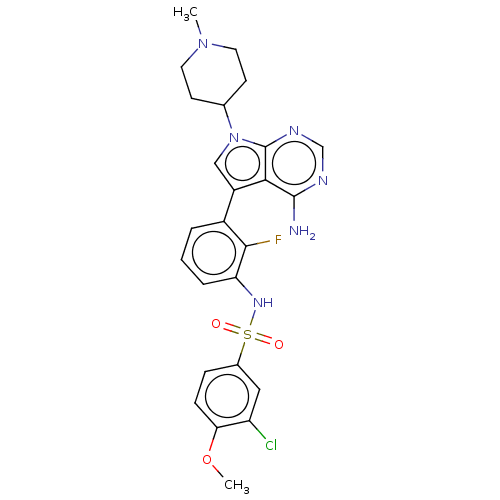
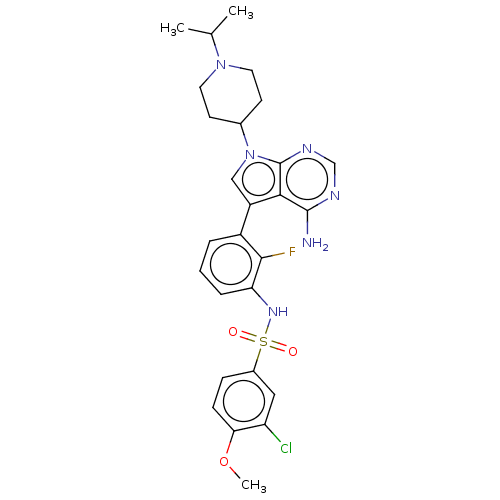
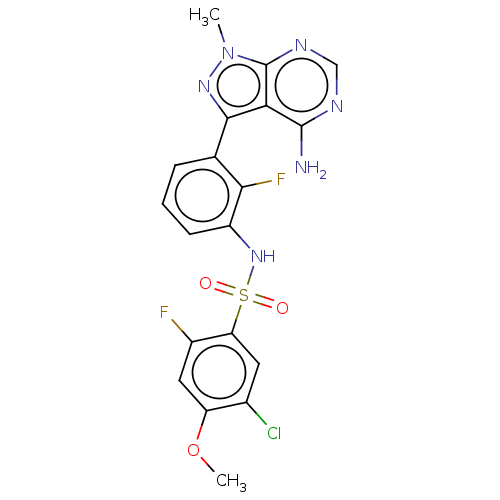
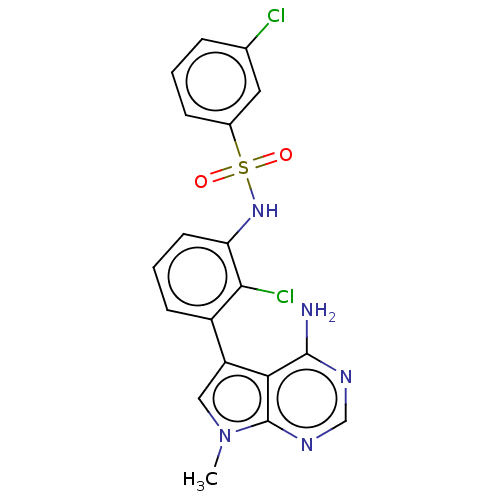
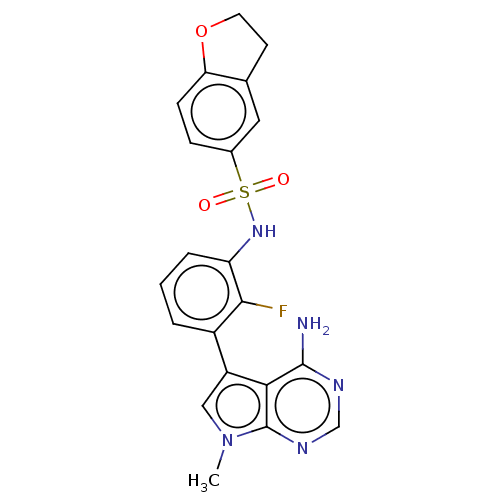
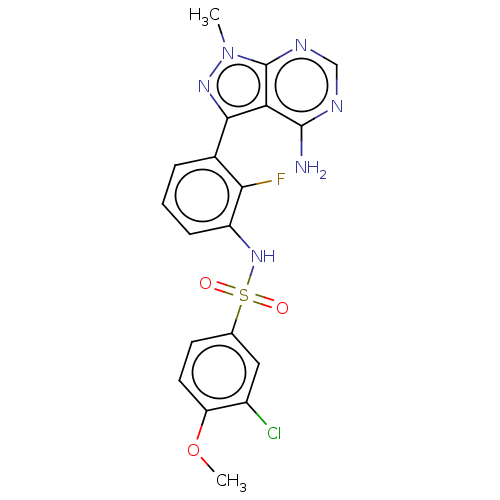
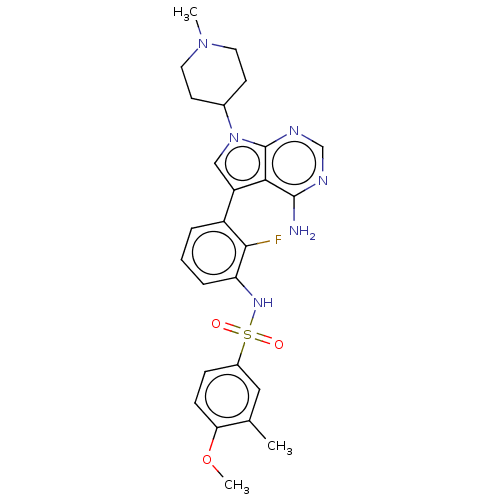
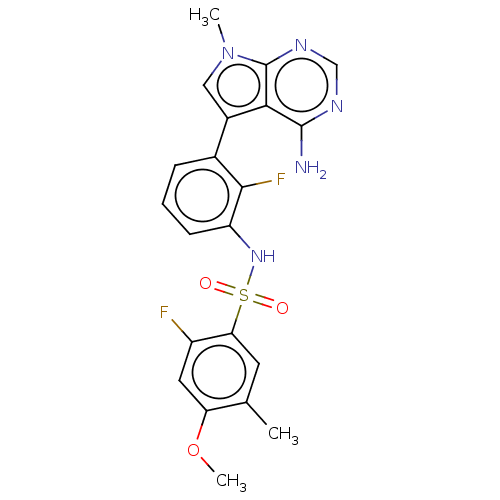
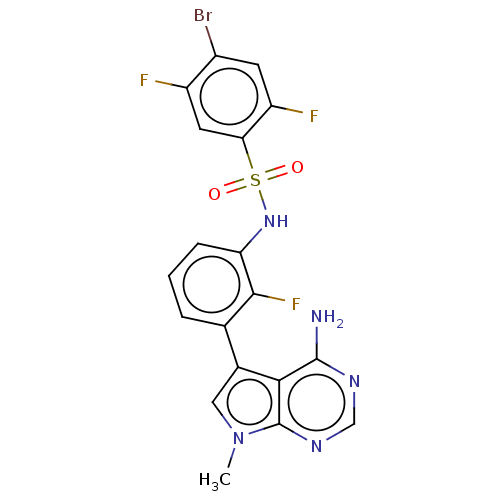
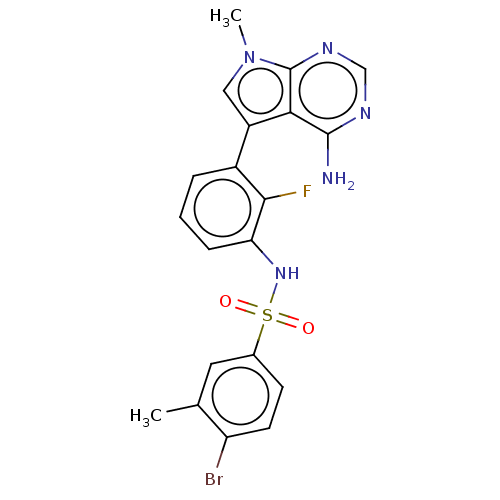
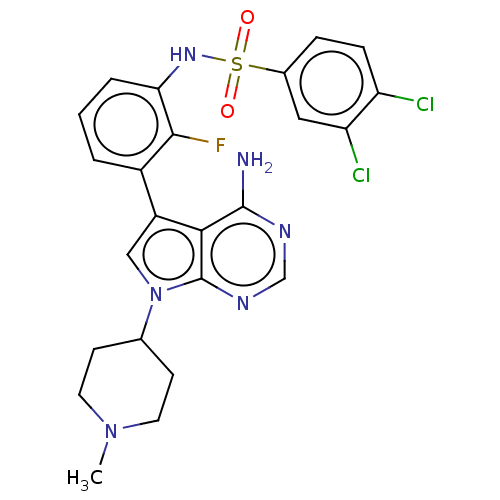
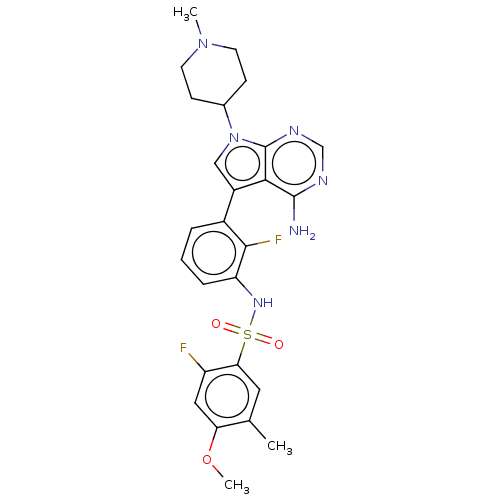
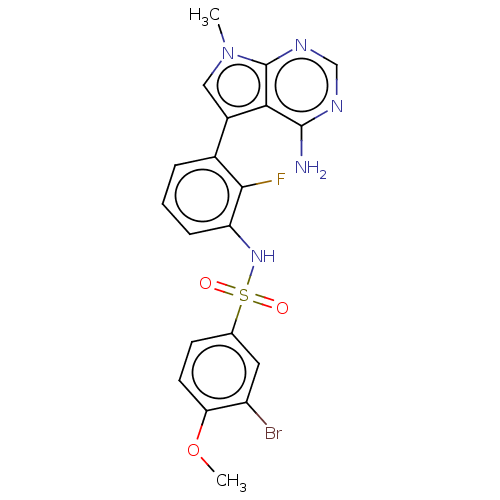
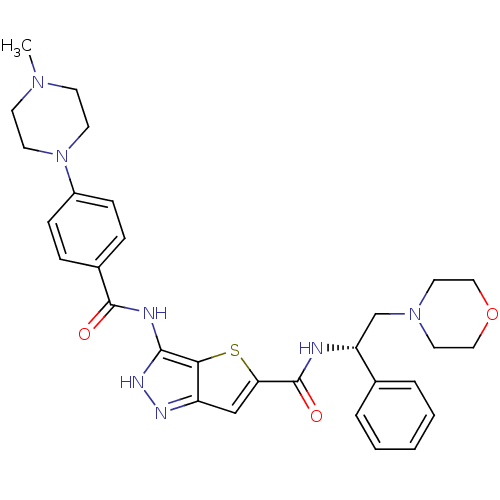
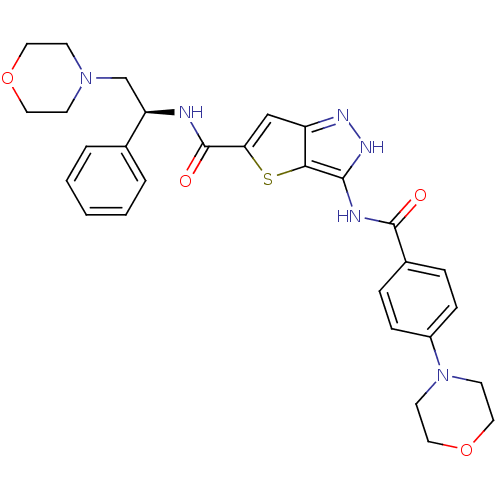
Affinity DataIC50: 3nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in...More data for this Ligand-Target Pair
TargetFibroblast growth factor receptor 1(Homo sapiens (Human))
Nerviano Medical Sciences Oncology
Curated by ChEMBL
Nerviano Medical Sciences Oncology
Curated by ChEMBL
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3 [540-1115](Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3 [540-1115](Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3 [540-1115](Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3 [540-1115](Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3 [540-1115](Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3 [540-1115](Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3 [540-1115](Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3 [540-1115](Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3 [540-1115](Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3 [540-1115](Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3 [540-1115](Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3 [540-1115](Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3 [540-1115](Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3 [540-1115](Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3 [540-1115](Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3 [540-1115](Homo sapiens (Human))
Nerviano Medical Sciences
US Patent
Nerviano Medical Sciences
US Patent
Affinity DataIC50: <16nMAssay Description:The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111...More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 3(Homo sapiens (Human))
Nerviano Medical Sciences Oncology
Curated by ChEMBL
Nerviano Medical Sciences Oncology
Curated by ChEMBL


 3D Structure (crystal)
3D Structure (crystal)