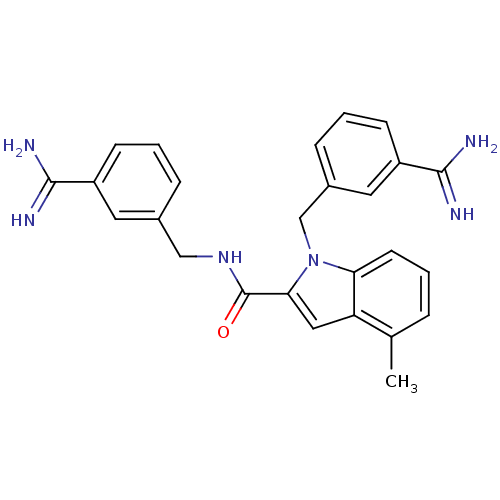
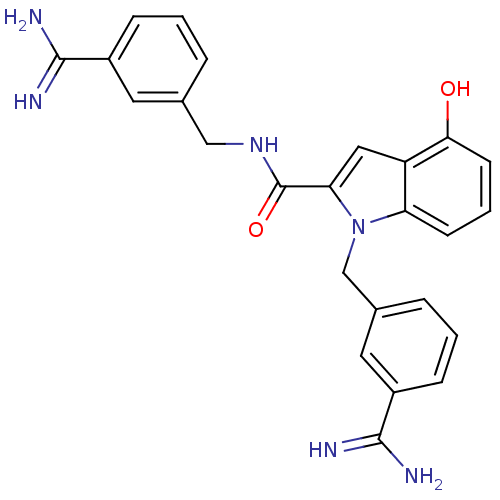
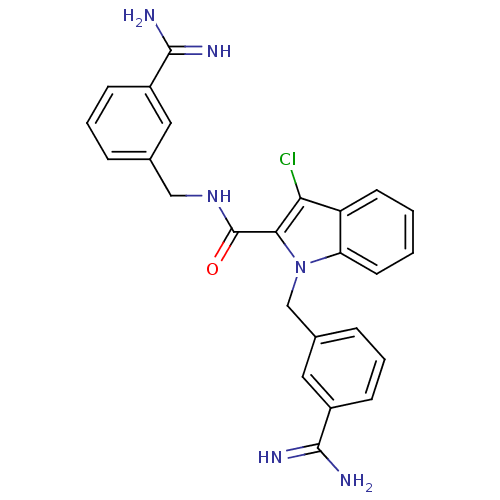
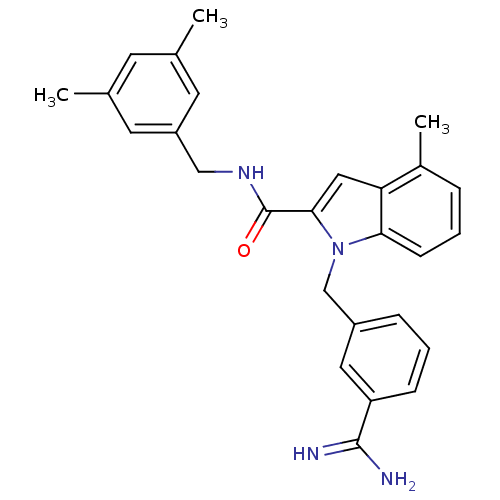
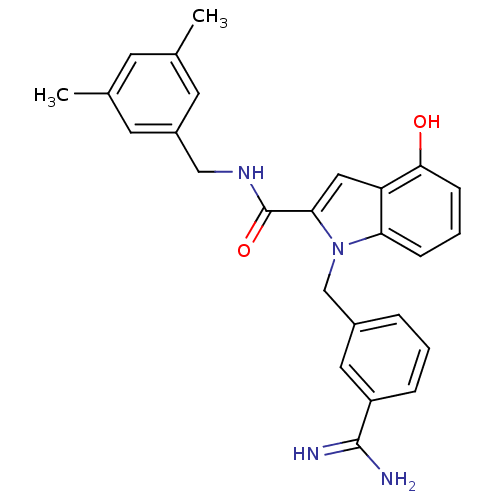
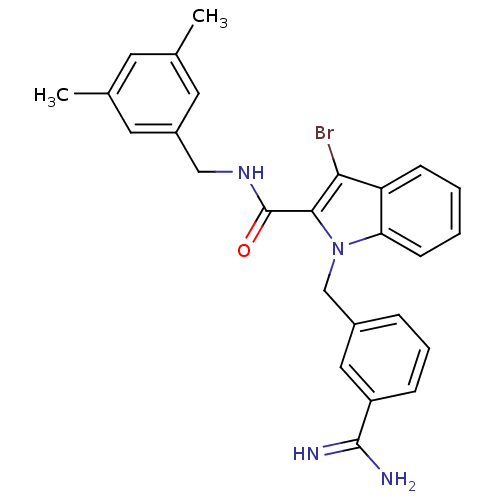
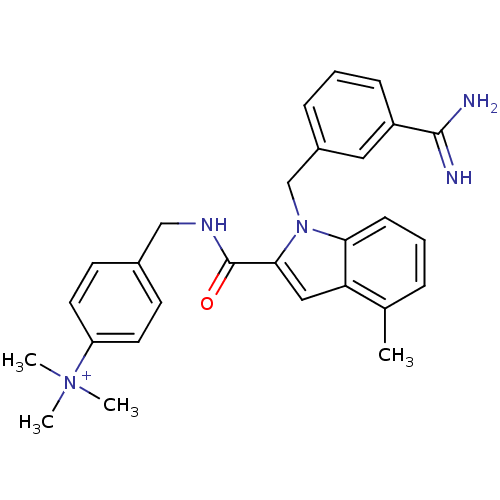
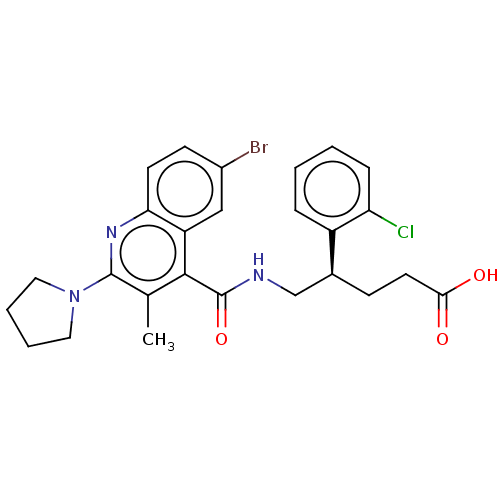
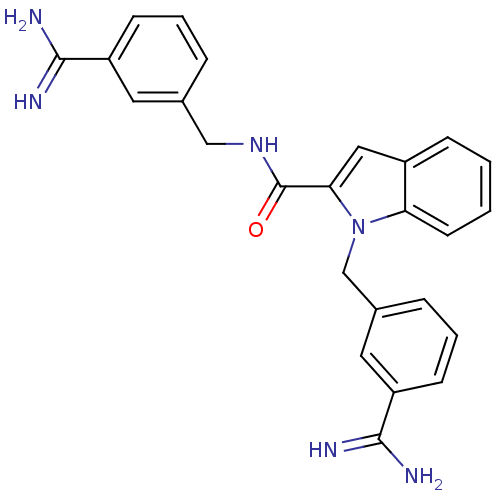
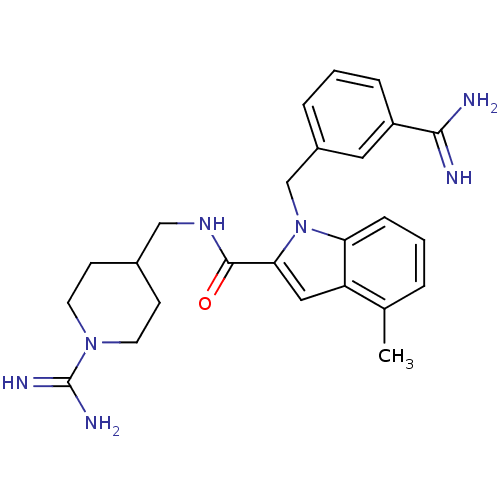
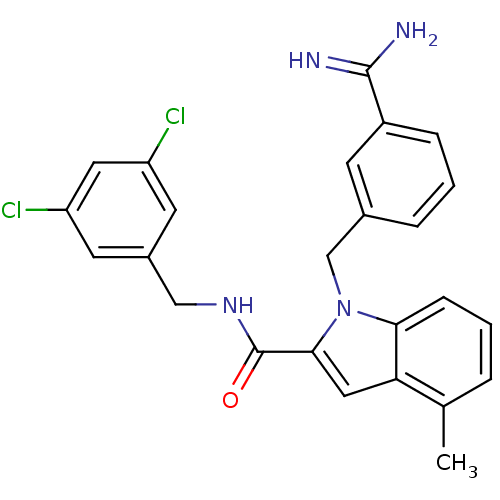
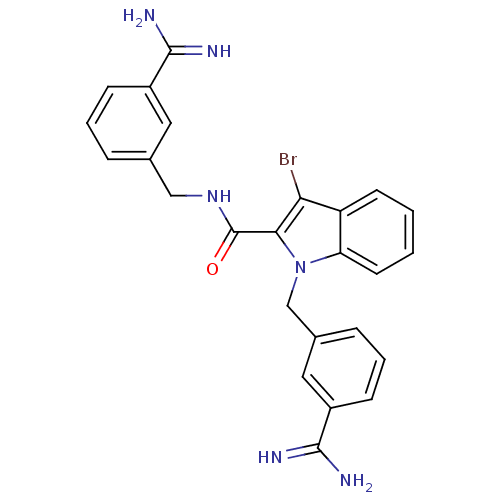
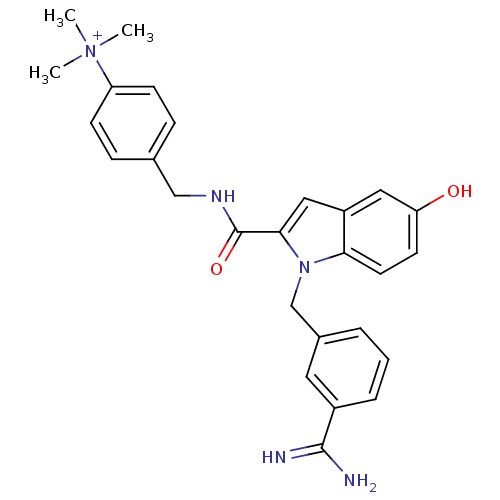
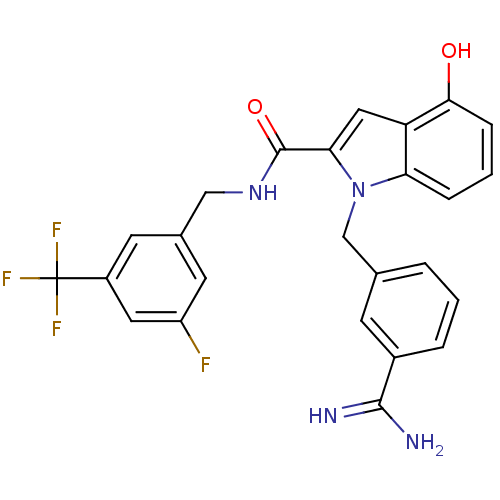
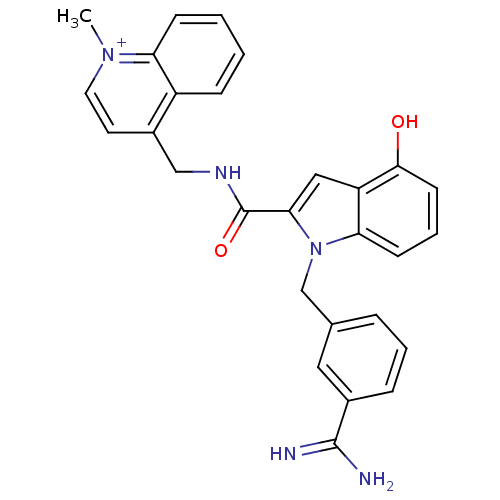
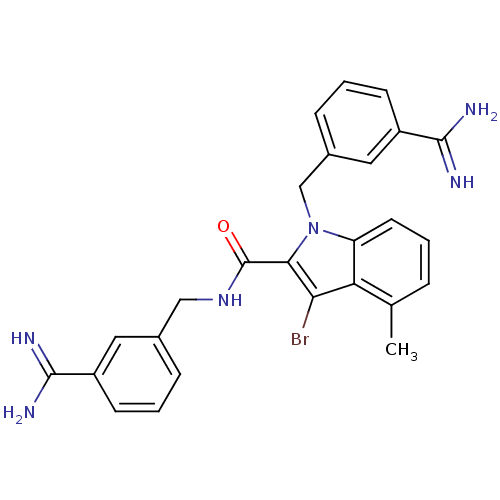
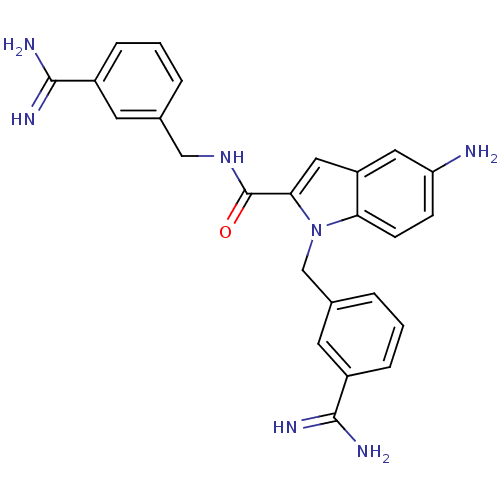
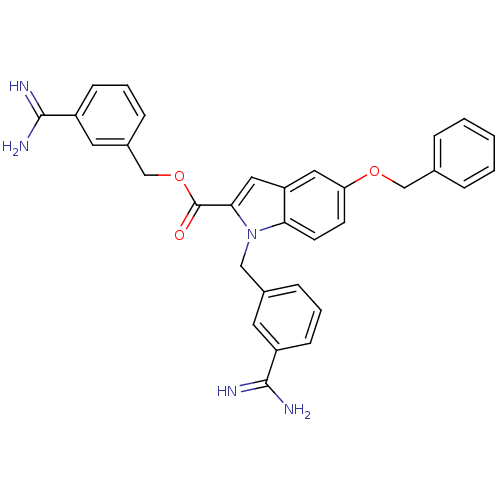
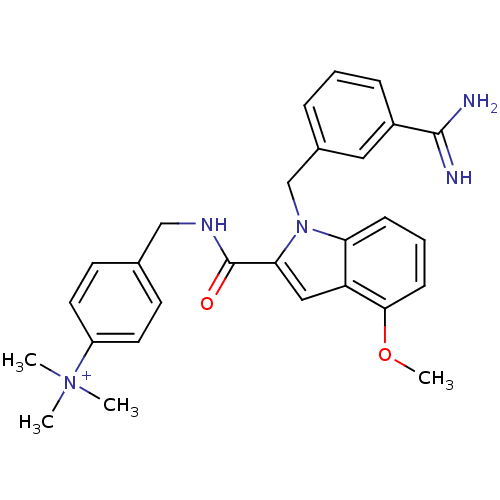
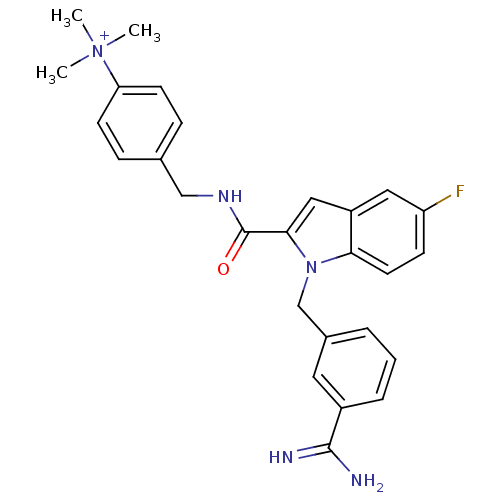
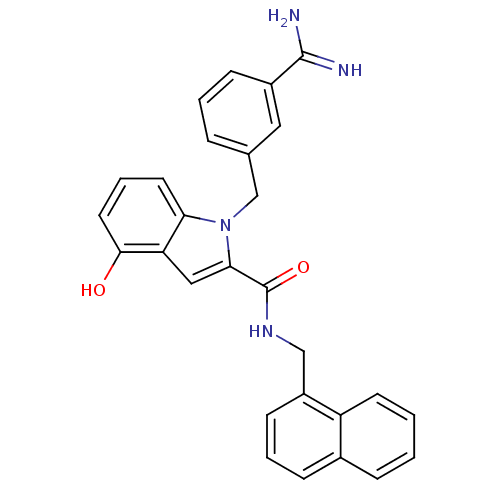
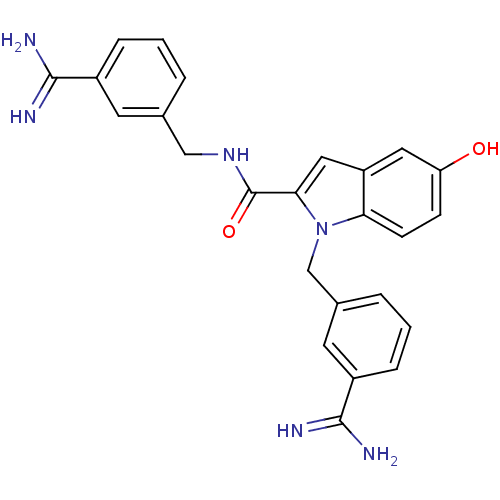
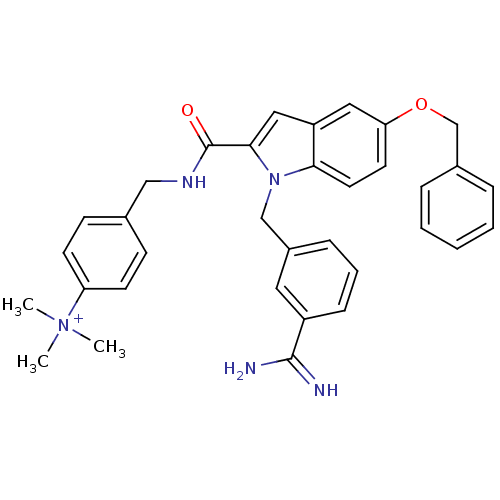
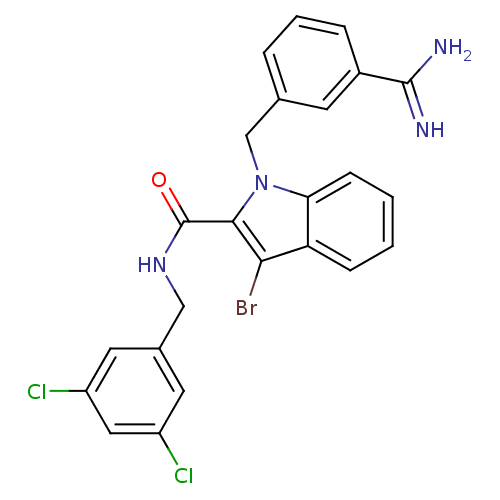
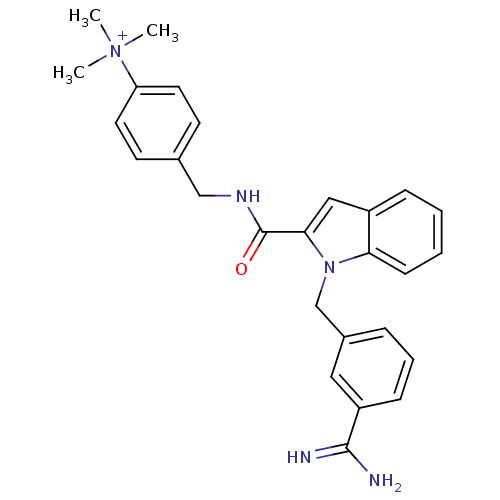
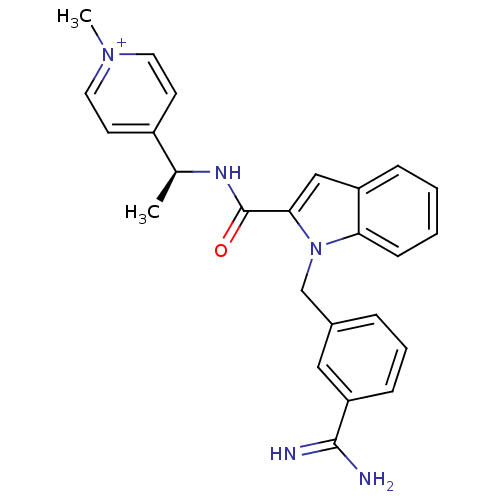
Affinity DataKi: 1nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 9nM ΔG°: -45.9kJ/molepH: 7.8 T: 2°CAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Evaluated for inhibitory activity against Human fidroblast collagenase (HFC)More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 13nM ΔG°: -45.0kJ/molepH: 7.8 T: 2°CAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Displacement of [3H]PGF2alpha from full-length recombinant human FP receptor expressed in HEK293 cell membranes measured after 60 mins by scintillati...More data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 26nM ΔG°: -43.3kJ/molepH: 7.8 T: 2°CAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 40nM ΔG°: -42.2kJ/molepH: 7.8 T: 2°CAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 42nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 50nM ΔG°: -41.7kJ/molepH: 7.8 T: 2°CAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 51nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 61nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 62nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 66nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 68nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 76nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 77nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 82nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 85nM ΔG°: -40.4kJ/molepH: 7.8 T: 2°CAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 90nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 92nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair





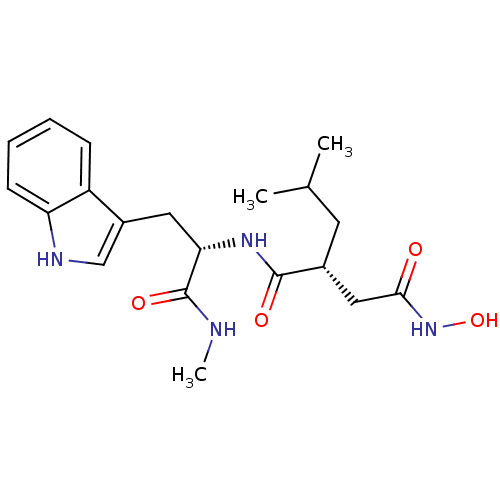









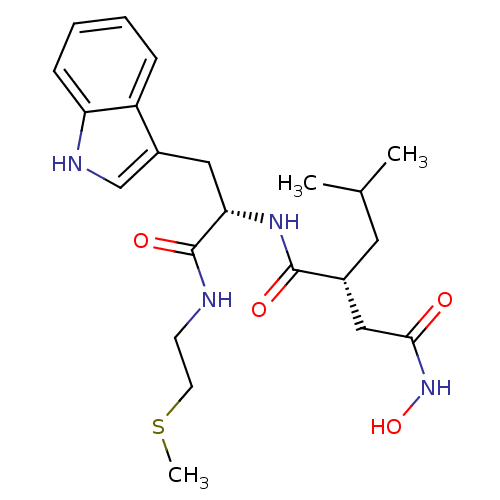


 3D Structure (crystal)
3D Structure (crystal)