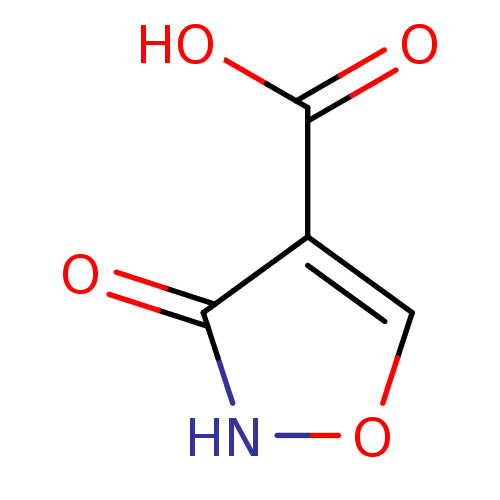
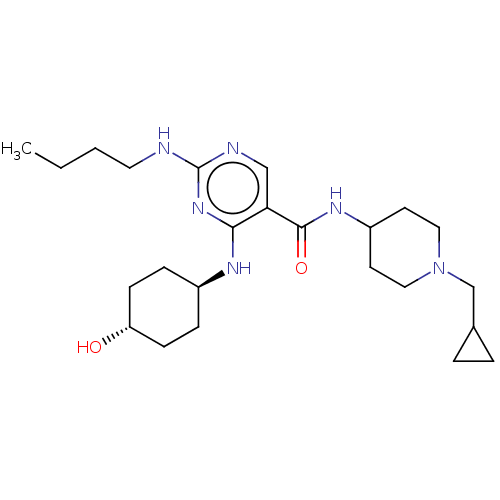
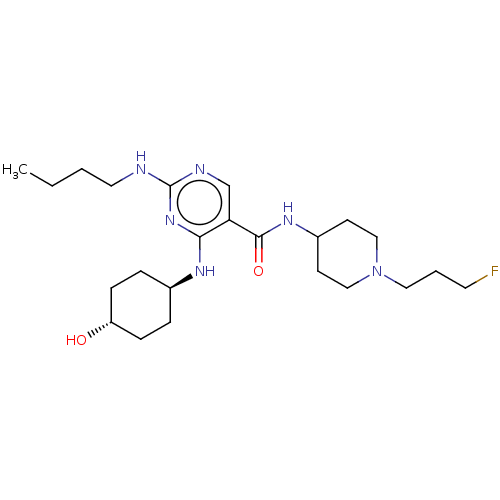
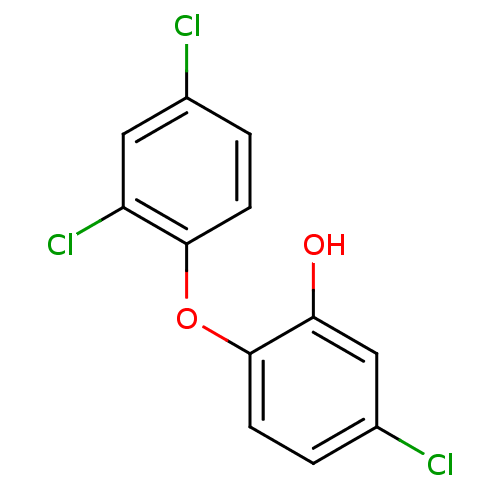
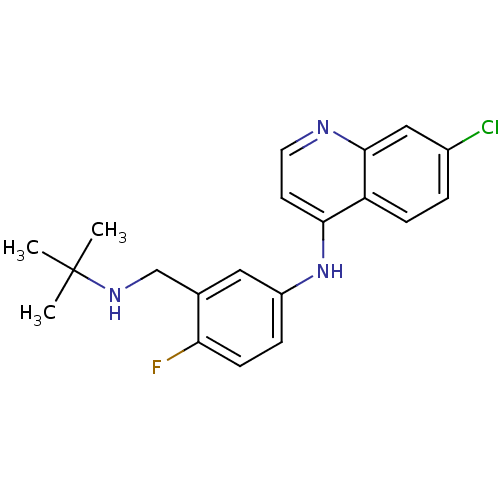
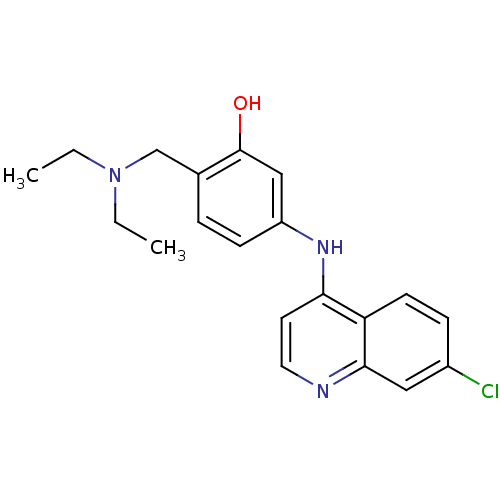
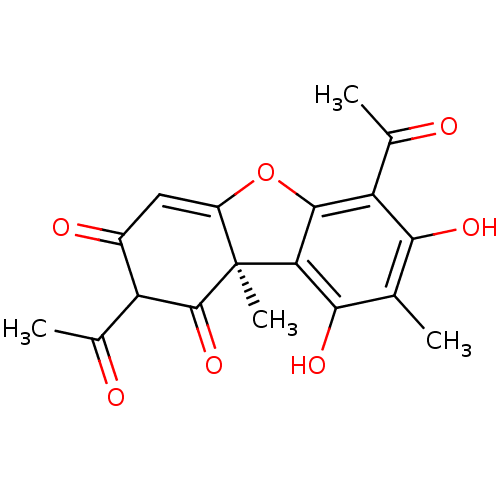
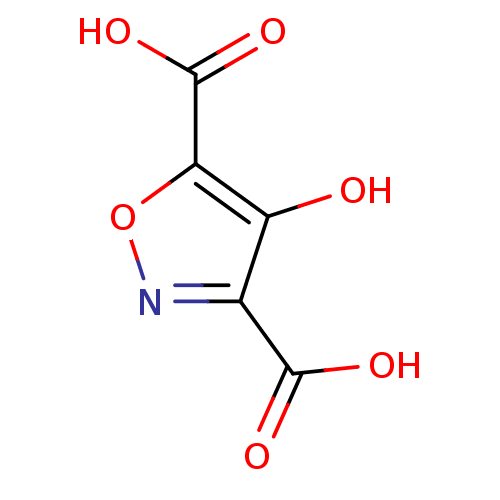
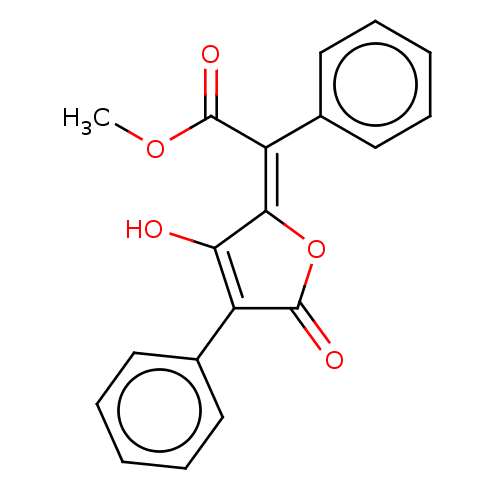
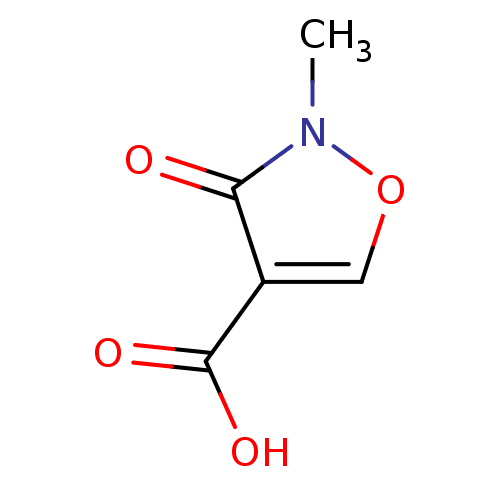
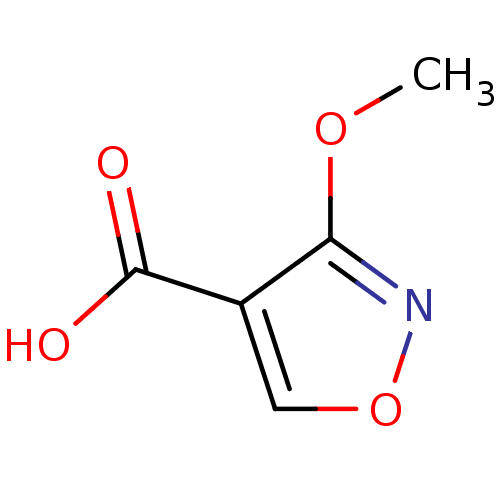
Affinity DataKi: 210nM ΔG°: -38.1kJ/mole IC50: 650nMpH: 7.5 T: 2°CAssay Description:An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou...More data for this Ligand-Target Pair
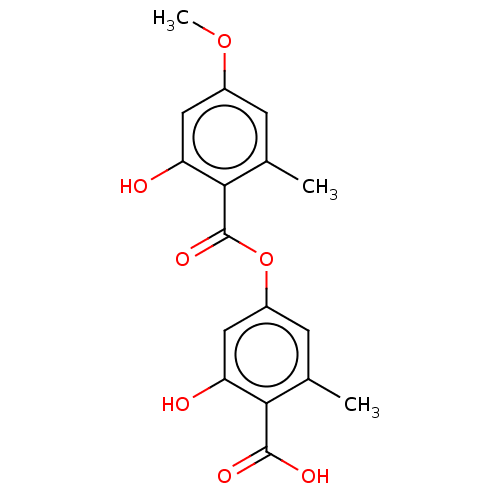
Affinity DataKi: 290nM ΔG°: -37.3kJ/mole IC50: 140nMpH: 7.5 T: 2°CAssay Description:An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou...More data for this Ligand-Target Pair
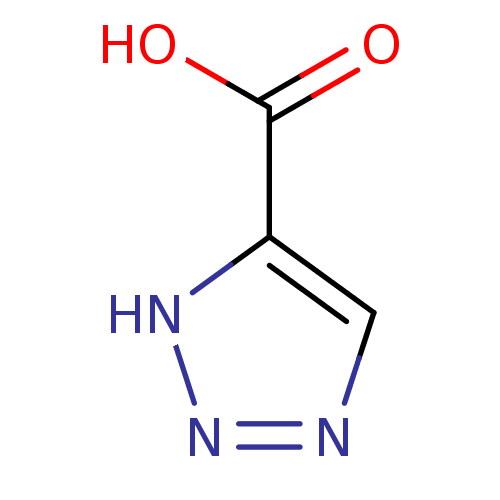
Affinity DataKi: 470nM ΔG°: -36.1kJ/mole IC50: 1.10E+3nMpH: 7.5 T: 2°CAssay Description:An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou...More data for this Ligand-Target Pair
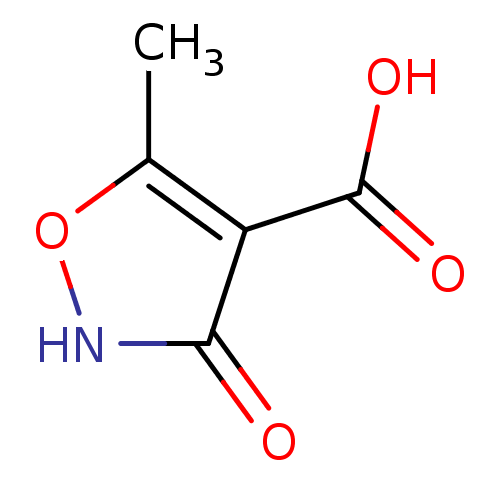
Affinity DataIC50: 3.60nMAssay Description:Inhibition of MERTK (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate incubated for 180 mins in presence of ATP by microfluidic capillary...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of MERTK (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate incubated for 180 mins in presence of ATP by microfluidic capillary...More data for this Ligand-Target Pair
Affinity DataIC50: 4.60nMAssay Description:Inhibition of MERTK (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate incubated for 180 mins in presence of ATP by microfluidic capillary...More data for this Ligand-Target Pair
TargetEnoyl-[acyl-carrier-protein] reductase [NADH] FabI(Escherichia coli)
University Of London
Curated by ChEMBL
University Of London
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of Escherichia coli FabI using 2-dodecenoyl-CoA as substrate at pH 8More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Monash University
Curated by ChEMBL
Monash University
Curated by ChEMBL
Affinity DataIC50: 49nMAssay Description:Inhibition of FLT3 (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate incubated for 180 mins in presence of ATP by microfluidic capillary el...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Monash University
Curated by ChEMBL
Monash University
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of FLT3 (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate incubated for 180 mins in presence of ATP by microfluidic capillary el...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Monash University
Curated by ChEMBL
Monash University
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibition of FLT3 (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate incubated for 180 mins in presence of ATP by microfluidic capillary el...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Monash University
Curated by ChEMBL
Monash University
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibition of Tyro3 (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate incubated for 180 mins in presence of ATP by microfluidic capillary...More data for this Ligand-Target Pair
Affinity DataIC50: 170nMAssay Description:Inhibition of Axl (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate incubated for 180 mins in presence of ATP by microfluidic capillary ele...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of Axl (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate incubated for 180 mins in presence of ATP by microfluidic capillary ele...More data for this Ligand-Target Pair
Affinity DataIC50: 218nMAssay Description:Inhibition of Axl (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate incubated for 180 mins in presence of ATP by microfluidic capillary ele...More data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Monash University
Curated by ChEMBL
Monash University
Curated by ChEMBL
Affinity DataIC50: 390nMAssay Description:Inhibition of Tyro3 (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate incubated for 180 mins in presence of ATP by microfluidic capillary...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Monash University
Curated by ChEMBL
Monash University
Curated by ChEMBL
Affinity DataIC50: 400nMAssay Description:Inhibition of Tyro3 (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate incubated for 180 mins in presence of ATP by microfluidic capillary...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of human CYP2C8More data for this Ligand-Target Pair
Affinity DataIC50: 6.10E+3nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 6.40E+3nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 8.80E+3nMAssay Description:Inhibition of human CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 9.80E+3nMAssay Description:Inhibition of human CYP2C8More data for this Ligand-Target Pair
Affinity DataIC50: 1.03E+4nMAssay Description:An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of human CYP2C8More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of human CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+4nMpH: 7.5 T: 2°CAssay Description:An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human CYP2C8More data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+4nMAssay Description:Inhibition of human CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+4nMAssay Description:Inhibition of human CYP3A4 using DEF substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+4nMAssay Description:Inhibition of human CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+4nMAssay Description:Inhibition of human CYP3A4 using DEF substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+4nMAssay Description:Inhibition of human CYP2C19More data for this Ligand-Target Pair
TargetEnoyl-[acyl-carrier-protein] reductase [NADH] FabI(Escherichia coli)
University Of London
Curated by ChEMBL
University Of London
Curated by ChEMBL
Affinity DataIC50: 4.70E+4nMAssay Description:Inhibition of Escherichia coli FabI using 2-dodecenoyl-CoA as substrate at pH 8More data for this Ligand-Target Pair
Affinity DataIC50: 5.20E+4nMAssay Description:Inhibition of human CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 5.40E+4nMAssay Description:Inhibition of human CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 5.40E+4nMAssay Description:An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou...More data for this Ligand-Target Pair
Affinity DataIC50: 5.80E+4nMAssay Description:Inhibition of human CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 6.20E+4nMAssay Description:Inhibition of human CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 6.40E+4nMAssay Description:Inhibition of human CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 7.21E+4nMAssay Description:An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou...More data for this Ligand-Target Pair
Affinity DataIC50: 7.50E+4nMAssay Description:Inhibition of human CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 8.10E+4nMpH: 7.5 T: 2°CAssay Description:An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou...More data for this Ligand-Target Pair
TargetEnoyl-[acyl-carrier-protein] reductase [NADH] FabI(Escherichia coli)
University Of London
Curated by ChEMBL
University Of London
Curated by ChEMBL
Affinity DataIC50: 8.40E+4nMAssay Description:Inhibition of Escherichia coli FabI using 2-dodecenoyl-CoA as substrate at pH 8More data for this Ligand-Target Pair
Affinity DataIC50: 8.50E+4nMAssay Description:Inhibition of human CYP2C19More data for this Ligand-Target Pair
TargetEnoyl-[acyl-carrier-protein] reductase [NADH] FabI(Escherichia coli)
University Of London
Curated by ChEMBL
University Of London
Curated by ChEMBL
Affinity DataIC50: 9.00E+4nMAssay Description:Inhibition of Escherichia coli FabI using 2-dodecenoyl-CoA as substrate at pH 8More data for this Ligand-Target Pair
Affinity DataIC50: 9.80E+4nMpH: 7.5 T: 2°CAssay Description:An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMpH: 7.5 T: 2°CAssay Description:An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMpH: 7.5 T: 2°CAssay Description:An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMpH: 7.5 T: 2°CAssay Description:An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)