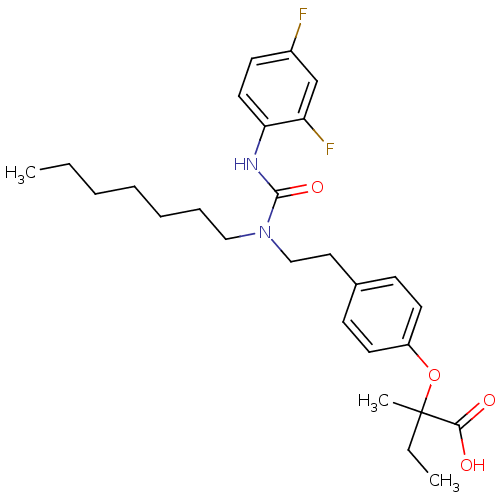
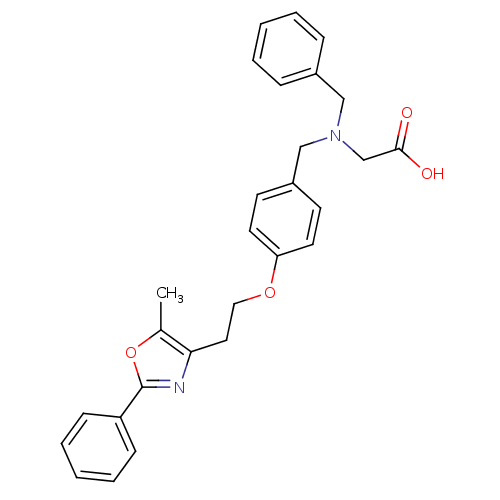
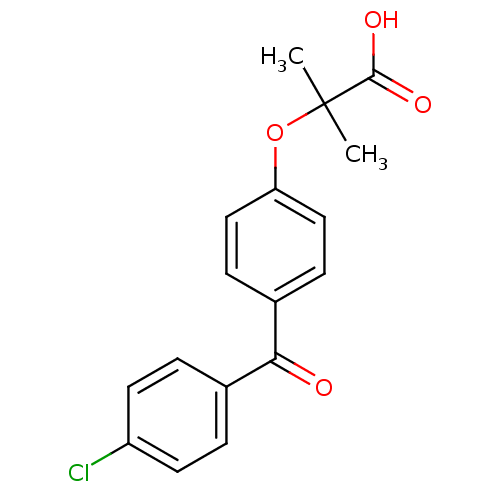
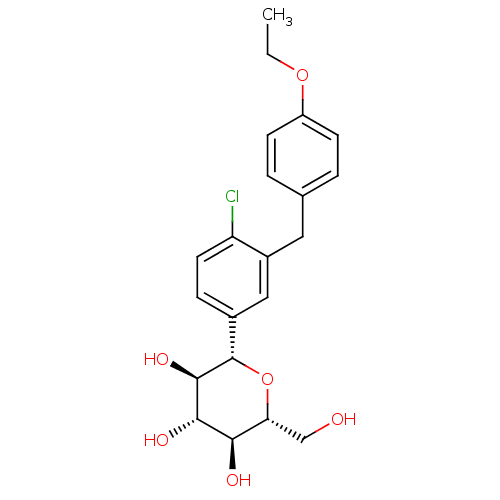
TargetMicrosomal triglyceride transfer protein large subunit(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0200nMAssay Description:In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assayMore data for this Ligand-Target Pair
TargetMicrosomal triglyceride transfer protein large subunit(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0300nMAssay Description:In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assayMore data for this Ligand-Target Pair
TargetMicrosomal triglyceride transfer protein large subunit(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0600nMAssay Description:In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assayMore data for this Ligand-Target Pair
TargetMicrosomal triglyceride transfer protein large subunit(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.150nMAssay Description:In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assayMore data for this Ligand-Target Pair
TargetMicrosomal triglyceride transfer protein large subunit(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.240nMAssay Description:In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assayMore data for this Ligand-Target Pair
TargetMicrosomal triglyceride transfer protein large subunit(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assayMore data for this Ligand-Target Pair
TargetMicrosomal triglyceride transfer protein large subunit(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay)More data for this Ligand-Target Pair
TargetMicrosomal triglyceride transfer protein large subunit(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay)More data for this Ligand-Target Pair
TargetMicrosomal triglyceride transfer protein large subunit(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay)More data for this Ligand-Target Pair
TargetMicrosomal triglyceride transfer protein large subunit(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay)More data for this Ligand-Target Pair
TargetMicrosomal triglyceride transfer protein large subunit(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay)More data for this Ligand-Target Pair
TargetMicrosomal triglyceride transfer protein large subunit(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:In vitro inhibition of human Microsomal Triglyceride Transfer Protein (triglyceride transfer assay)More data for this Ligand-Target Pair
TargetMicrosomal triglyceride transfer protein large subunit(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:In vitro inhibition of human microsomal triglyceride transfer protein using triglyceride transfer assayMore data for this Ligand-Target Pair
TargetMicrosomal triglyceride transfer protein large subunit(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay)More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of human Peroxisome proliferator activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:Inhibition of human Peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:Inhibition of human Peroxisome proliferator activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 316nMAssay Description:Inhibition of human Peroxisome proliferator activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 538nMAssay Description:Inhibition of human Peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of human Peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.80E+3nMAssay Description:Inhibition of human Peroxisome proliferator activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human Peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: >2.50E+4nMAssay Description:Inhibition of human Peroxisome proliferator activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human Peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Effective concentration against human Peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 110nMAssay Description:Effective concentration against human Peroxisome proliferator activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Effective concentration against human Peroxisome proliferator activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: >3.20E+4nMAssay Description:Effective concentration against human Peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 320nMAssay Description:Effective concentration against human Peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 140nMAssay Description:Effective concentration against human Peroxisome proliferator activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 1.50E+3nMAssay Description:Effective concentration against human Peroxisome proliferator activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 425nMAssay Description:Effective concentration against human Peroxisome proliferator activated receptor gammaMore data for this Ligand-Target Pair
Affinity DataEC50: 1.39E+3nMpH: 7.2 T: 2°CAssay Description:Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt...More data for this Ligand-Target Pair
Affinity DataEC50: >8.00E+3nMpH: 7.2 T: 2°CAssay Description:Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt...More data for this Ligand-Target Pair
Affinity DataEC50: >8.00E+3nMpH: 7.2 T: 2°CAssay Description:Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt...More data for this Ligand-Target Pair
Affinity DataEC50: 211nMpH: 7.2 T: 2°CAssay Description:Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt...More data for this Ligand-Target Pair
Affinity DataEC50: 330nMpH: 7.2 T: 2°CAssay Description:Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt...More data for this Ligand-Target Pair
Affinity DataEC50: 1.10nMpH: 7.2 T: 2°CAssay Description:Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt...More data for this Ligand-Target Pair
Affinity DataEC50: 1.30E+3nMpH: 7.2 T: 2°CAssay Description:Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt...More data for this Ligand-Target Pair
Affinity DataEC50: 9.20nMpH: 7.2 T: 2°CAssay Description:Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 20nMAssay Description:Effective concentration against human Peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 35.6nMpH: 7.2 T: 2°CAssay Description:Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt...More data for this Ligand-Target Pair
Affinity DataEC50: 6.60nMpH: 7.2 T: 2°CAssay Description:Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt...More data for this Ligand-Target Pair

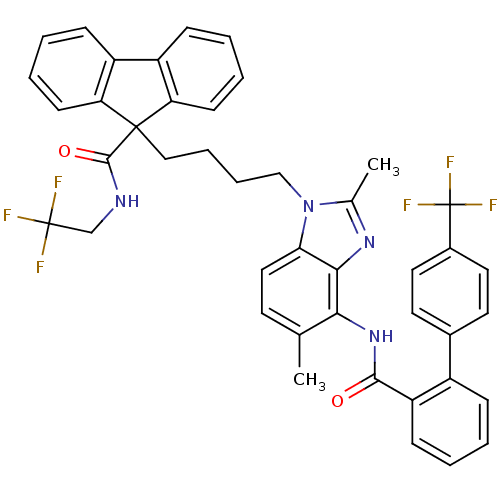



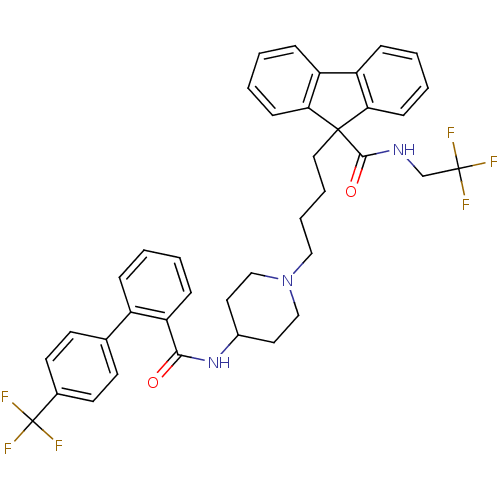

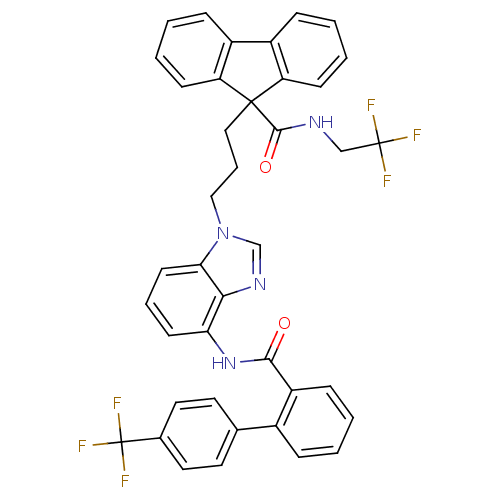
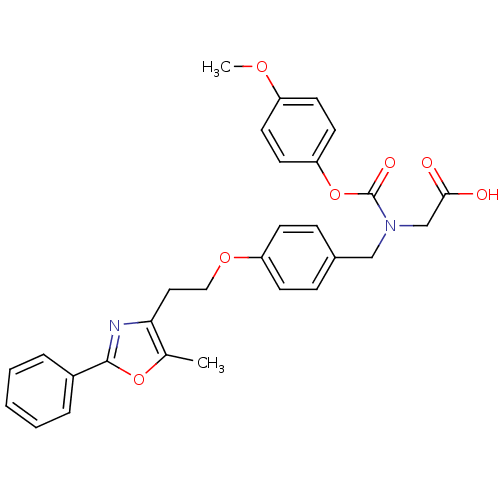

 3D Structure (crystal)
3D Structure (crystal)