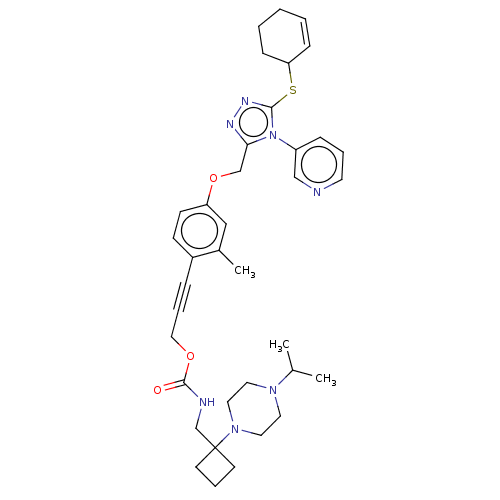
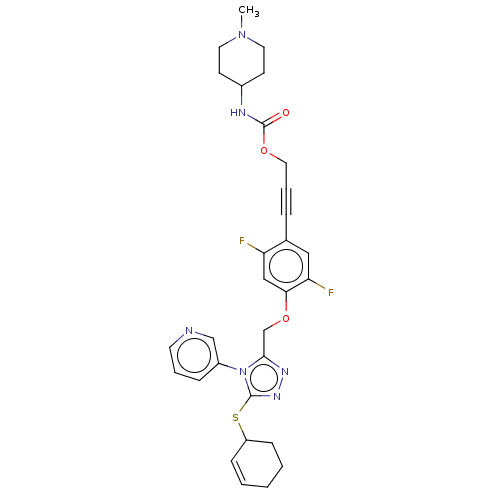
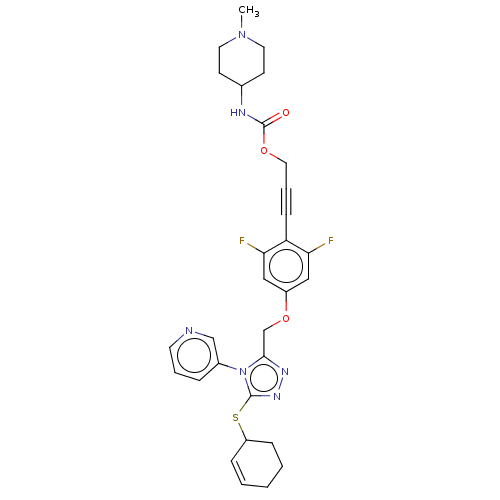
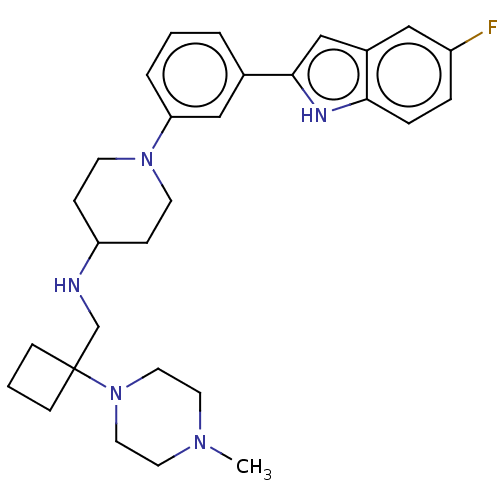
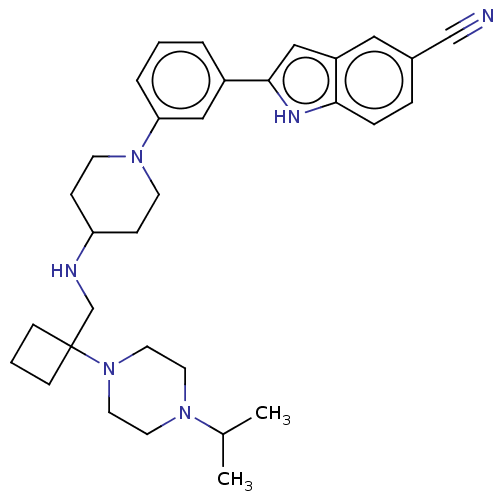
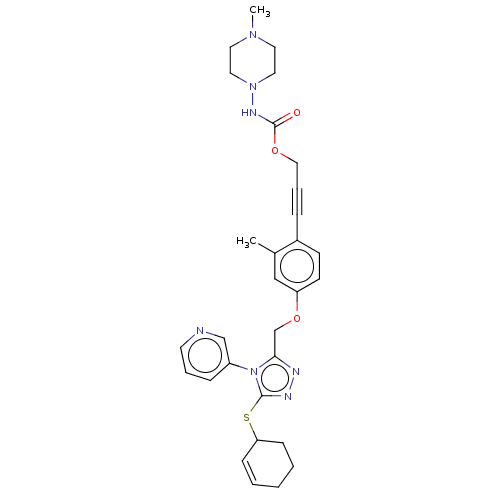
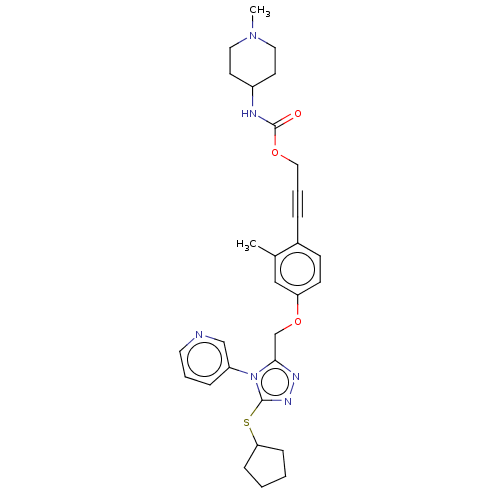
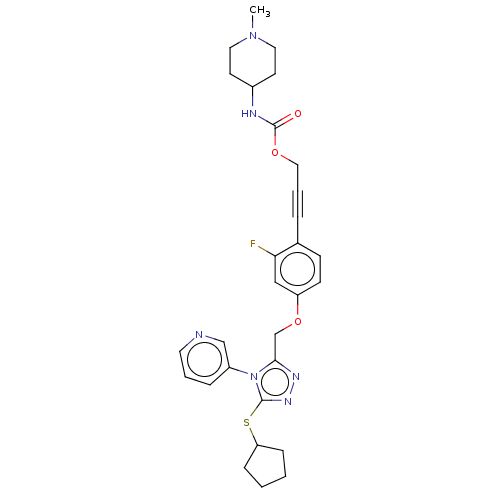
Affinity DataKi: 5.20nMAssay Description:Inhibition of PARP1 (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
TargetG-protein coupled estrogen receptor 1(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataKi: 5.70nMAssay Description:Inhibition of estrogen binding to GPR30 (unknown origin)More data for this Ligand-Target Pair
TargetG-protein coupled estrogen receptor 1(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Inhibition of estrogen binding to GPR30 (unknown origin)More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 300nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 300nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
Affinity DataKi: 572nMAssay Description:Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre...More data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assayMore data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assayMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataKi: 740nMAssay Description:Uncompetitive inhibition of recombinant human full length Avi-tagged p97 (1 to 806 residues) expressed in Escherichia coli Rosetta 2(DE3) using 20 uM...More data for this Ligand-Target Pair
Affinity DataKi: 900nMAssay Description:Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre...More data for this Ligand-Target Pair
Affinity DataKi: 1.90E+3nMAssay Description:Competitive inhibition of His6-tagged human recombinant Cdc25B catalytic domain (350 to 566 residues) expressed in Escherichia coli BL21 (D3) after 2...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
Affinity DataKi: 2.12E+3nMAssay Description:Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataKi: 3.50E+3nMAssay Description:Competitive inhibition of recombinant human full length Avi-tagged p97 (1 to 806 residues) expressed in Escherichia coli Rosetta 2(DE3) using 20 uM A...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
Affinity DataKi: 4.60E+3nMAssay Description:In vitro inhibitory activity against human recombinant Cdc25B phosphatase enzymeMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 6.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 8.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
Affinity DataKi: 8.52E+3nMAssay Description:Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assayMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 3.00E+4nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+4nMAssay Description:Inhibitory activity against human recombinant Cdc25B phosphatase enzymeMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 6.00E+4nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 6.00E+4nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: >3.00E+5nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30nMAssay Description:Inhibition of PARP1 (unknown origin)More data for this Ligand-Target Pair
TargetSignal peptide peptidase-like 2A(Homo sapiens)
The Genomics Institute of The Novartis Research Foundation
Curated by ChEMBL
The Genomics Institute of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human SPPL2a expressed in HEK293 cells using GAL4-VP16 fusedTNFalpha (1 to 76)-NTF as substrate after 24 hrs by Bright-Glo luciferase r...More data for this Ligand-Target Pair
TargetSignal peptide peptidase-like 2A(Homo sapiens)
The Genomics Institute of The Novartis Research Foundation
Curated by ChEMBL
The Genomics Institute of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of human SPPL2a expressed in HEK293 cells using GAL4-VP16 fusedTNFalpha (1 to 76)-NTF as substrate after 24 hrs by Bright-Glo luciferase r...More data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the...More data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of p97 (unknown origin) assessed as reduction in ATPase activity by biomol green reagent based assayMore data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of p97 (unknown origin) assessed as reduction in ATPase activity by biomol green reagent based assayMore data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the...More data for this Ligand-Target Pair
TargetSignal peptide peptidase-like 2A(Homo sapiens)
The Genomics Institute of The Novartis Research Foundation
Curated by ChEMBL
The Genomics Institute of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of human SPPL2a expressed in HEK293 cells using GAL4-VP16 fusedTNFalpha (1 to 76)-NTF as substrate after 24 hrs by Bright-Glo luciferase r...More data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of recombinant human full length Avi-tagged p97 (1 to 806 residues) expressed in Escherichia coli Rosetta 2(DE3) using 100 uM ATP as subst...More data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the...More data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the...More data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the...More data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the...More data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass...More data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass...More data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the...More data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the...More data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the...More data for this Ligand-Target Pair

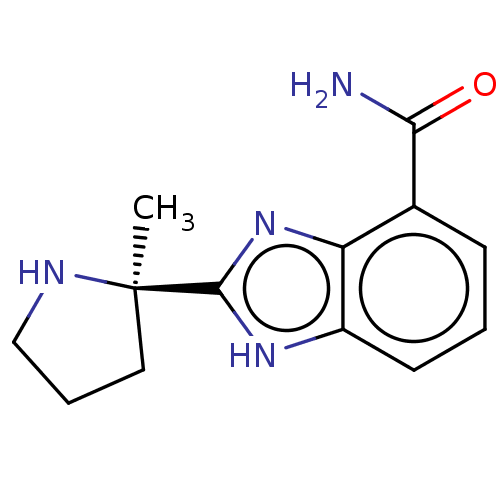
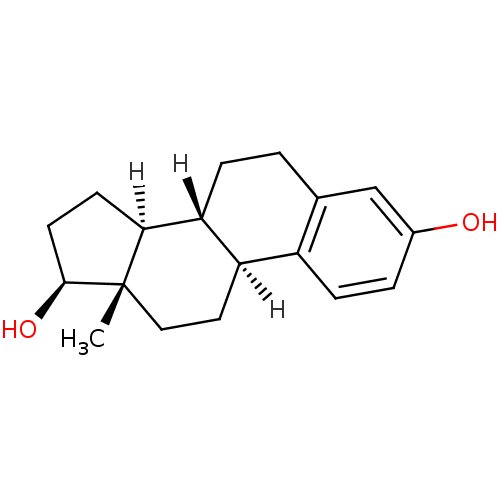

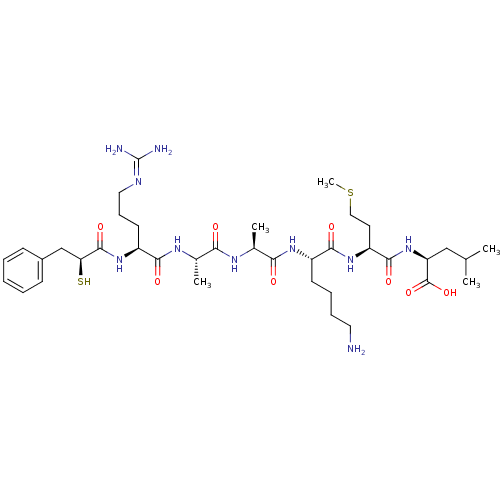











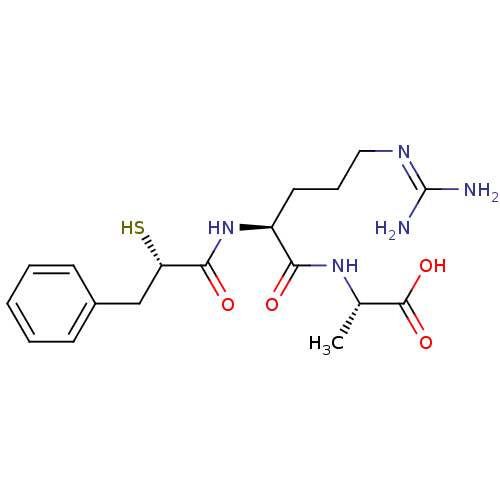
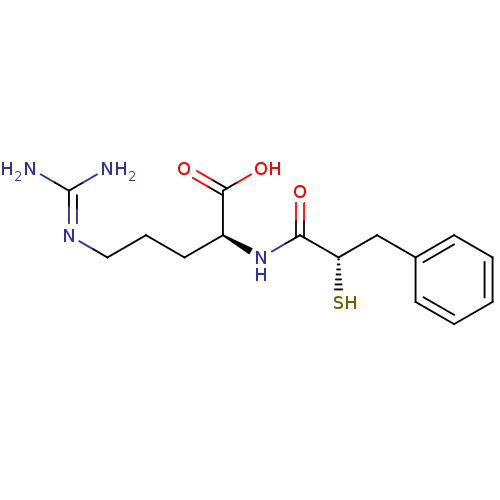




 3D Structure (crystal)
3D Structure (crystal)