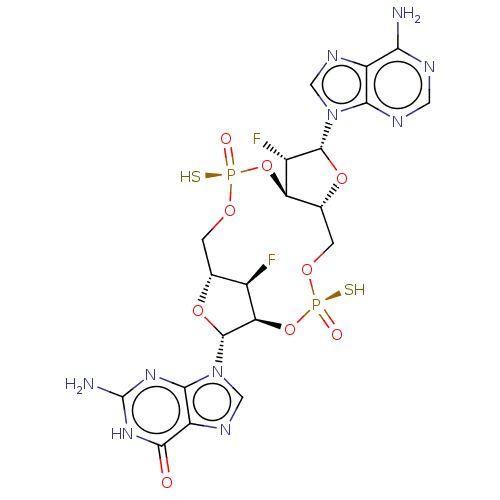
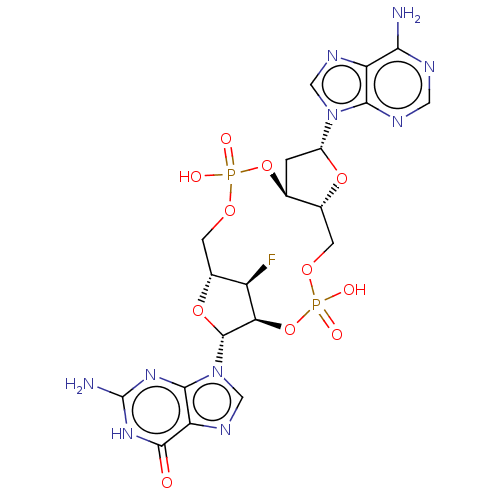
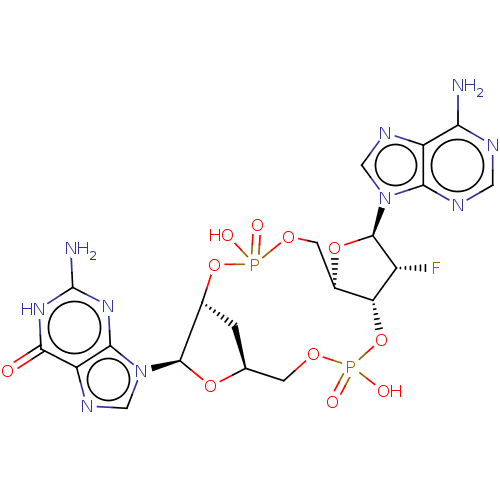
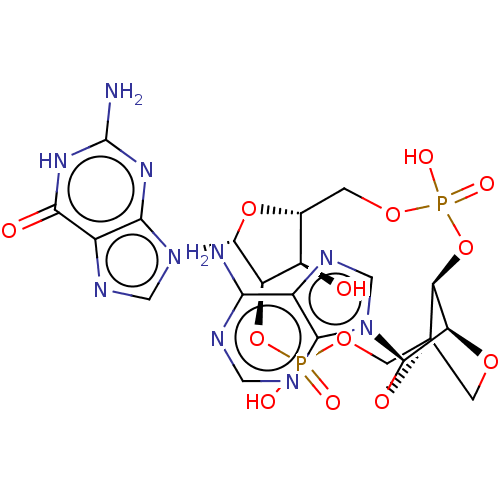
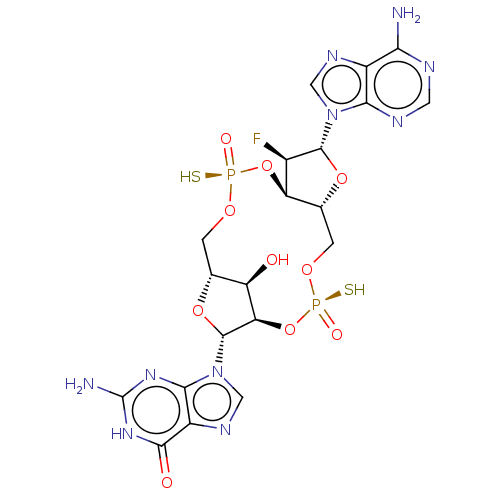
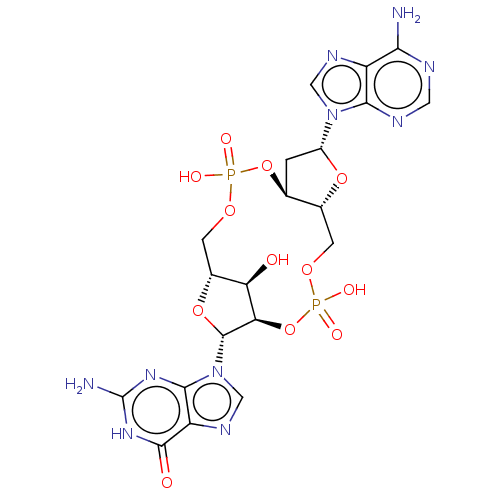
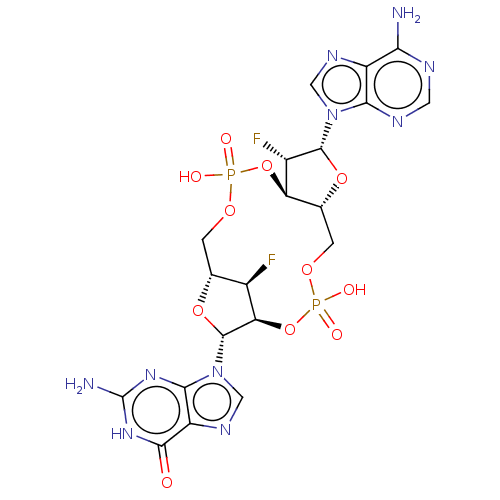
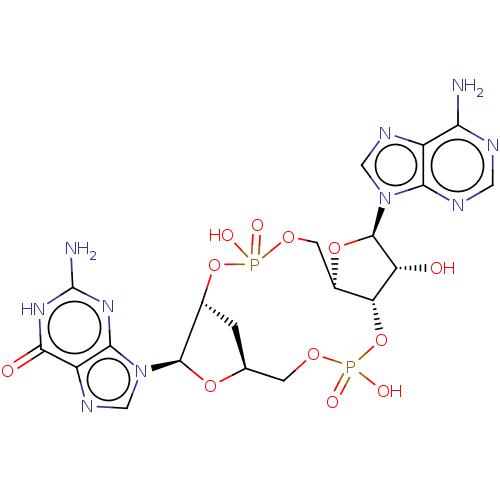
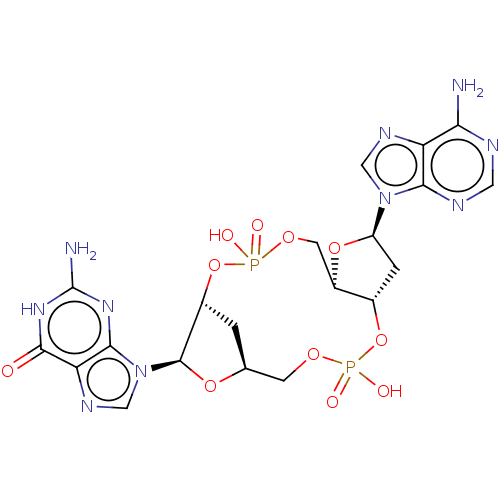
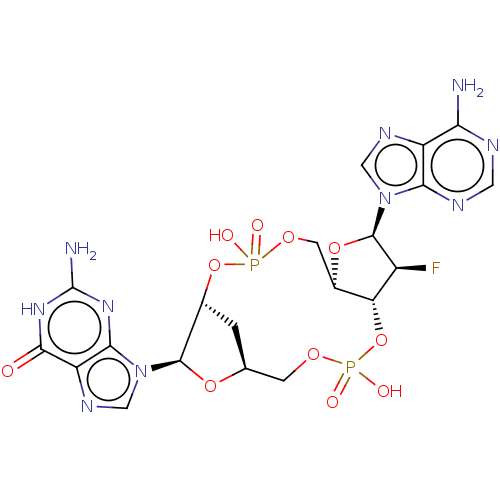
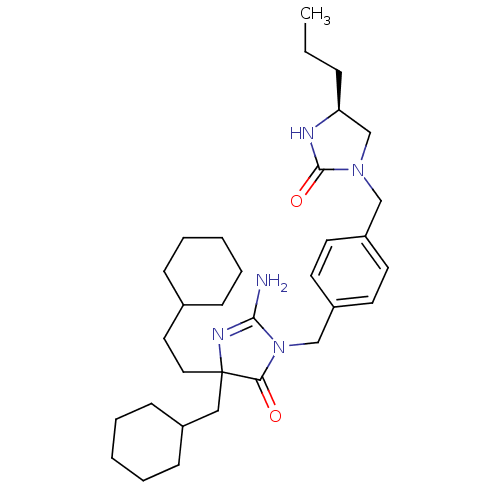
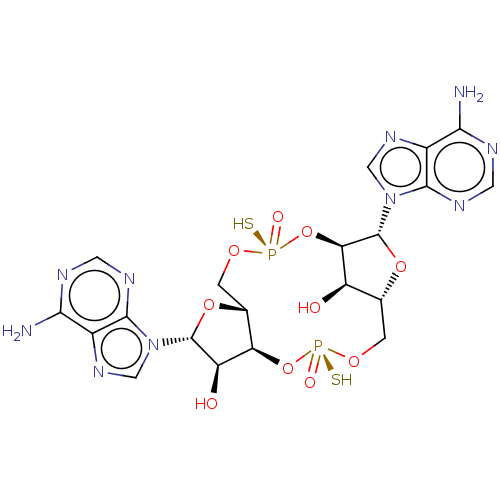
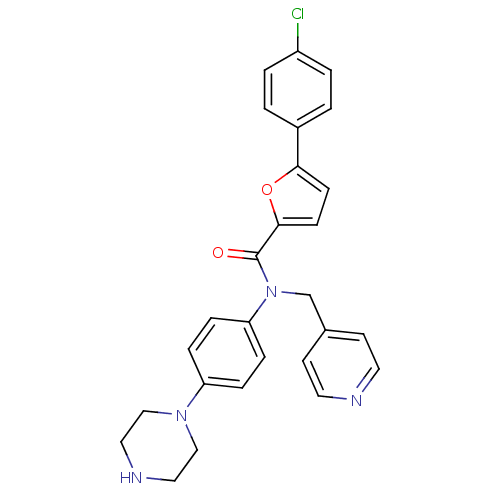
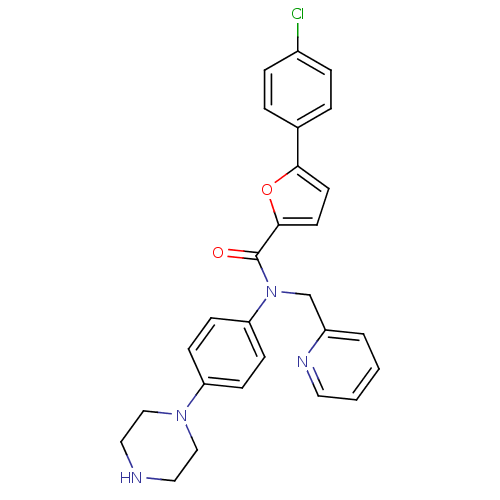
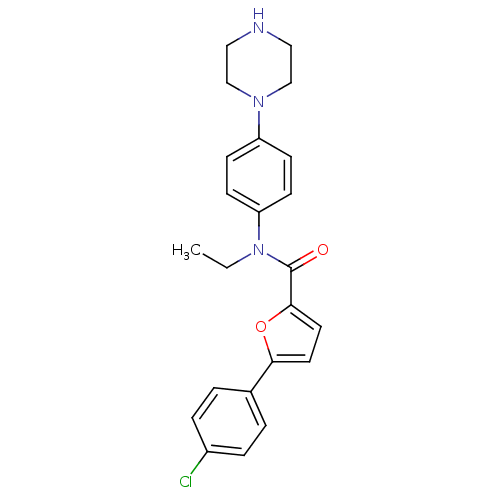
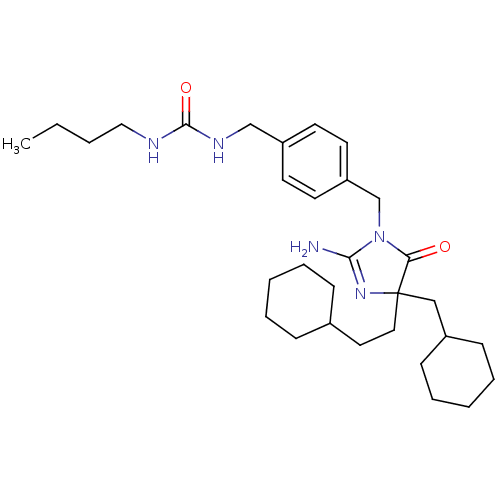
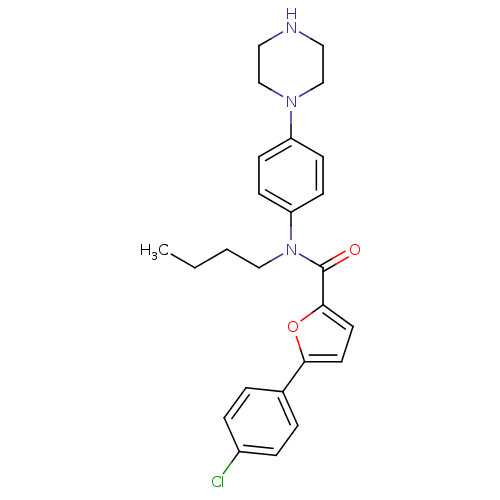
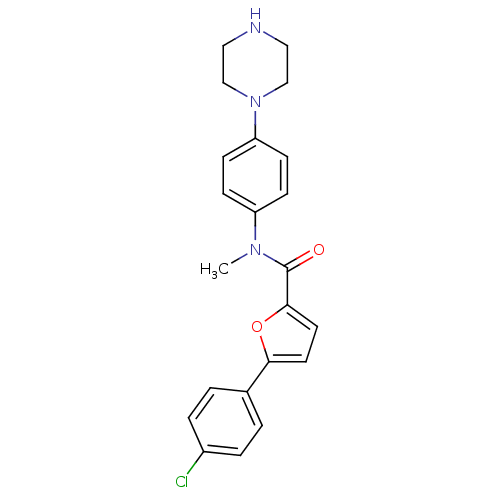
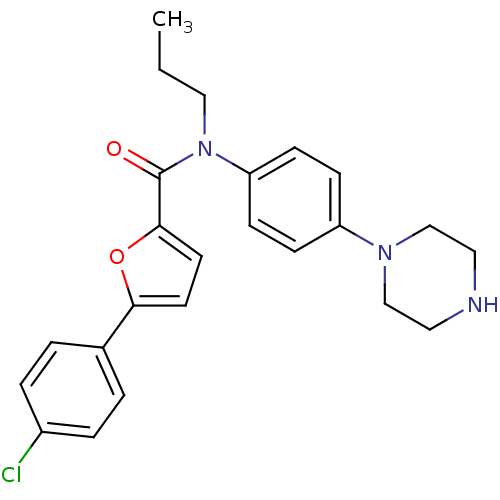
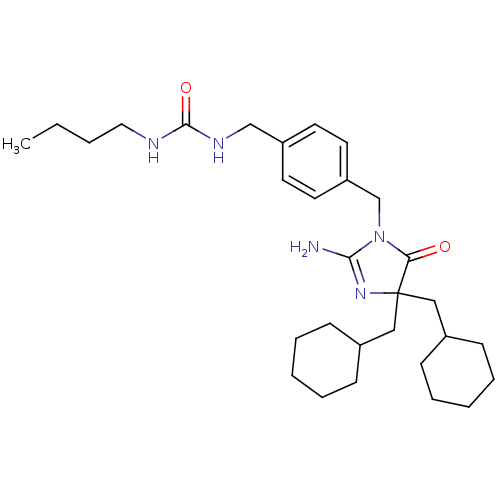
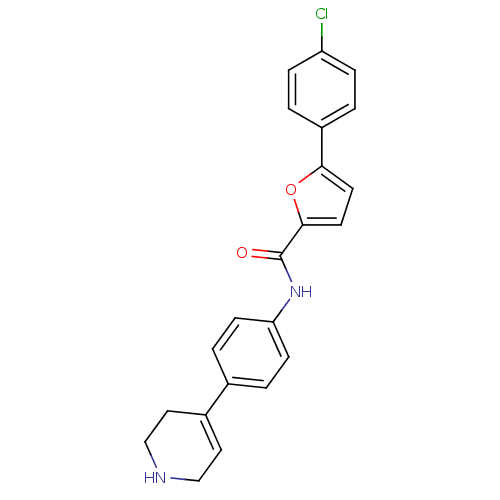
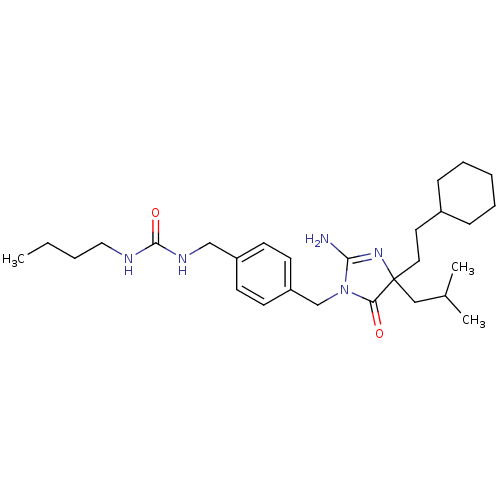
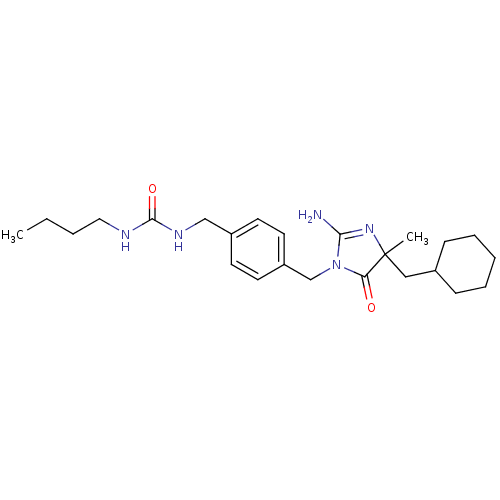
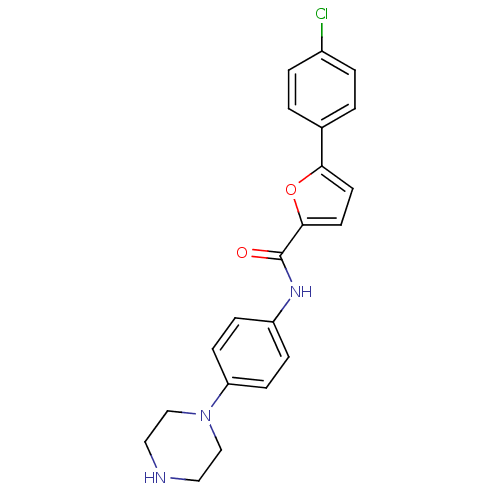
Affinity DataKi: 740nMAssay Description:Inhibition of human cathepsin D preincubated for 30 minsMore data for this Ligand-Target Pair
Affinity DataKi: 800nMAssay Description:Inhibition constant for HCV NS3 protease substrate binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition constant for HCV NS3 protease substrate binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 8.00E+4nMAssay Description:Inhibition constant for HCV NS3 protease substrate binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+5nMAssay Description:Inhibition constant for HCV NS3 protease substrate binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+5nMAssay Description:Inhibition constant for HCV NS3 protease substrate binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 1.80E+5nMAssay Description:Inhibition constant for HCV NS3 protease substrate binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 2.10E+5nMAssay Description:Inhibition constant for HCV NS3 protease substrate binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 2.60E+5nMAssay Description:Inhibition constant for HCV NS3 protease substrate binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 3.10E+5nMAssay Description:Inhibition constant for HCV NS3 protease substrate binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 6.00E+5nMAssay Description:Inhibition constant for HCV NS3 protease substrate binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 6.50E+5nMAssay Description:Inhibition constant for HCV NS3 protease substrate binding siteMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.20nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.30nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibition of BACE1 mediated human Swedish amyloid precursor protein peptide hydrolysis by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Displacement of radiolabeled [3H]-2',3'-cGAMP from wild type human STING incubated for 2 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 90nMAssay Description:Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA...More data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA...More data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA...More data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:Inhibition of BACE1 mediated human Swedish amyloid precursor protein peptide hydrolysis by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 440nMAssay Description:Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA...More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA...More data for this Ligand-Target Pair
Affinity DataIC50: 605nMAssay Description:Inhibition of BACE1 mediated human Swedish amyloid precursor protein peptide hydrolysis by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 650nMAssay Description:Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of BACE1 mediated human Swedish amyloid precursor protein peptide hydrolysis by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of BACE1 mediated hydrolysis of human amyloid precursor protein with Swedish and London mutation in HEK293 cells by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of BACE1 mediated human Swedish amyloid precursor protein peptide hydrolysis by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of BACE1 mediated human Swedish amyloid precursor protein peptide hydrolysis by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 2 uM ATP by DELFIA a...More data for this Ligand-Target Pair

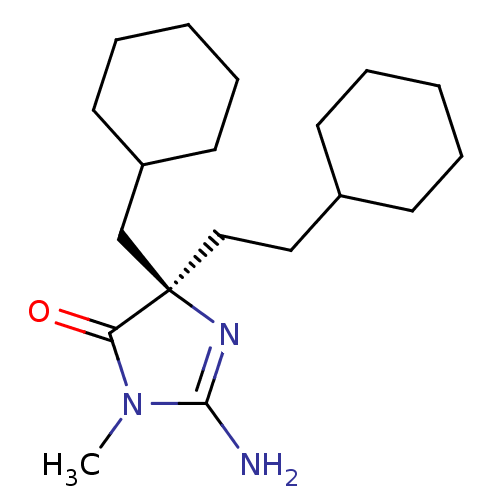
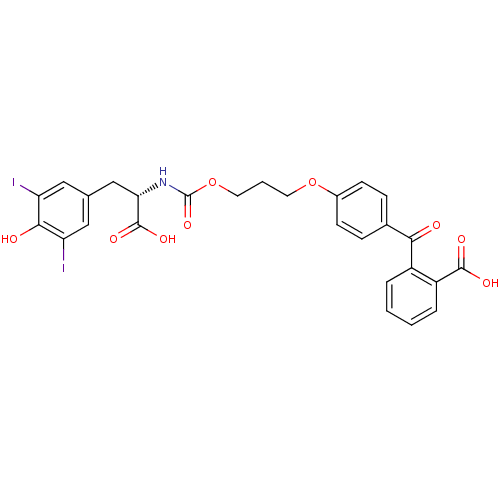


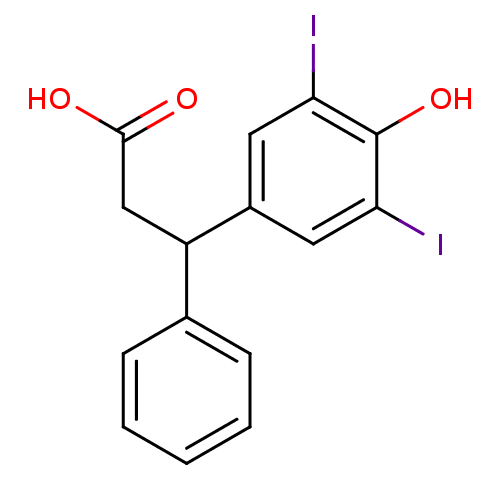

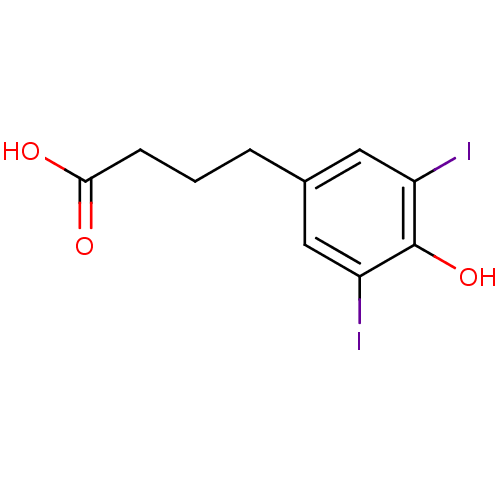

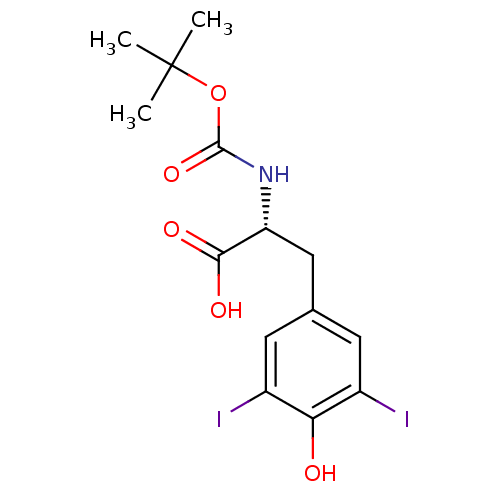



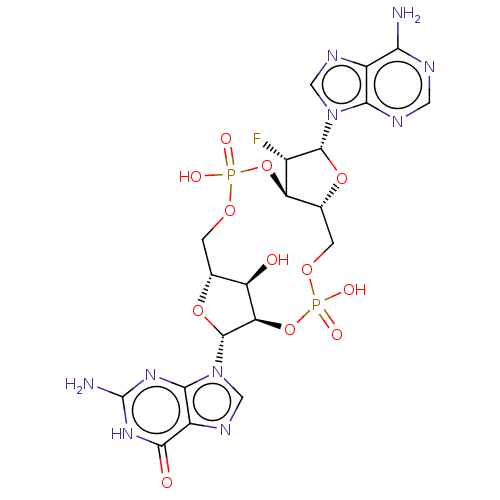
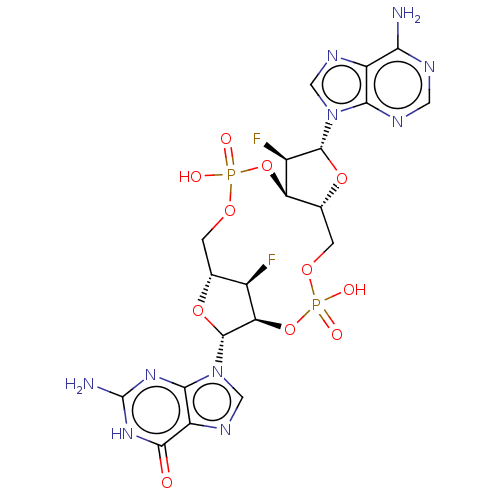
 3D Structure (crystal)
3D Structure (crystal)