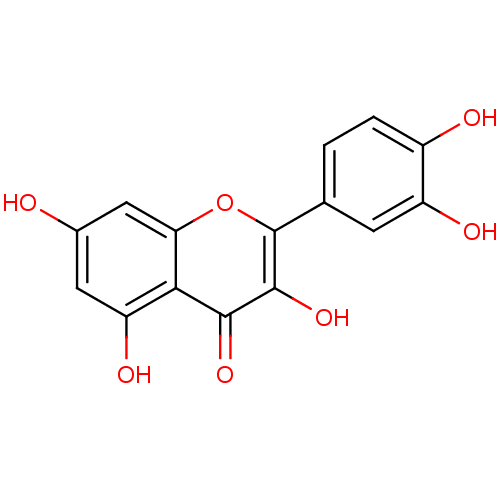
BDBM7460 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one::2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-chromen-4-one::2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-chromone;hydrate::CHEMBL50::Quercetin::Quercetin (10)::Quercetin (21)::Quercetin (Qur)::US11021454, Compound Quercetin::US9180183, Quercetin::med.21724, Compound 4
SMILES Oc1cc(O)c2c(c1)oc(-c1ccc(O)c(O)c1)c(O)c2=O
InChI Key InChIKey=REFJWTPEDVJJIY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 7460
Found 9 hits for monomerid = 7460
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Mrc
Mrc
Affinity DataIC50: 3.80E+3nM Kd: 280nMpH: 7.2 T: 2°CAssay Description:Binding was detected as a change in the intrinsic tryptophan fluorescence of the PI3K upon the addition of inhibitor. The inhibitor was incubated wit...More data for this Ligand-Target Pair
TargetIsoform A1-A of Heterogeneous nuclear ribonucleoprotein A1 (A1-A)(Homo sapiens (Human))
National Taiwan University
National Taiwan University
Affinity DataKd: 8.90E+3nMpH: 4.0Assay Description:Recombinant full-length hnRNPA1 (aa 1-320) and truncated versions of hnRNPA1, including the N-terminal RNA binding domain (aa 1-196), the middle regi...More data for this Ligand-Target Pair
TargetIsoform A1-A of Heterogeneous nuclear ribonucleoprotein A1 (A1-A) 68-320](Homo sapiens (Human))
National Taiwan University
National Taiwan University
Affinity DataKd: 1.70E+3nMpH: 4.0Assay Description:Recombinant full-length hnRNPA1 (aa 1-320) and truncated versions of hnRNPA1, including the N-terminal RNA binding domain (aa 1-196), the middle regi...More data for this Ligand-Target Pair
Affinity DataKd: 38.7nMAssay Description:Inhibition of human thrombin assessed as equilibrium dissociation constant at 50 to 1000 uM by BIAcore analysisMore data for this Ligand-Target Pair
Affinity DataKd: 25nMAssay Description:Binding affinity to non phosphorylated PIM1More data for this Ligand-Target Pair
Affinity DataKd: 7.08E+3nMAssay Description:Binding affinity to ABCB1 nucleotide binding domain 2More data for this Ligand-Target Pair
Affinity DataKd: 1.29E+4nMAssay Description:Binding affinity to Helicobacter pylori Ddl by surface plasmon resonance biosensor technologyMore data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
National Research Council (Itb-Cnr)
Curated by ChEMBL
National Research Council (Itb-Cnr)
Curated by ChEMBL
Affinity DataKd: 2.56E+4nMAssay Description:Binding affinity to sensorchip-immobilized human His-tagged CFTR F508 deletion mutant by surface plasmon resonance analysisMore data for this Ligand-Target Pair
Affinity DataKd: 5.18E+4nMAssay Description:Binding affinity to human MMP-1 catalytic domain (100 to 269 residues) expressed in Escherichia coli BL21 Star (DE3) by SPR analysisMore data for this Ligand-Target Pair
Activity Spreadsheet -- ITC Data from BindingDB
 Found 4 hits for monomerid = 7460
Found 4 hits for monomerid = 7460
ITC DataΔG°: -9.78kcal/mole −TΔS°: -0.257kcal/mole ΔH°: -9.58kcal/mole logk: 3.93E+7
pH: 7.5 T: 10.00°C
pH: 7.5 T: 10.00°C
ITC DataΔG°: -8.08kcal/mole −TΔS°: 0.840kcal/mole ΔH°: -8.92kcal/mole logk: 6.82E+5
pH: 7.0 T: 30.00°C
pH: 7.0 T: 30.00°C
ITC DataΔG°: -7.40kcal/mole −TΔS°: -2.66kcal/mole ΔH°: -4.74kcal/mole logk: 2.20E+5
pH: 7.0 T: 30.00°C
pH: 7.0 T: 30.00°C
ITC DataΔG°: -7.94kcal/mole −TΔS°: -2.82kcal/mole ΔH°: -5.12kcal/mole logk: 5.42E+5
pH: 7.0 T: 30.00°C
pH: 7.0 T: 30.00°C
 3D Structure (crystal)
3D Structure (crystal)