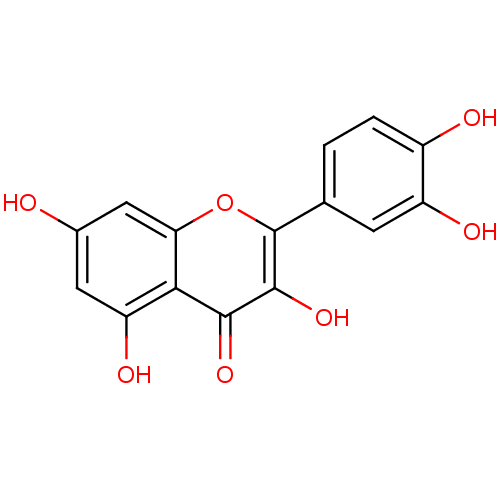
BDBM7460 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one::2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-chromen-4-one::2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-chromone;hydrate::CHEMBL50::Quercetin::Quercetin (10)::Quercetin (21)::Quercetin (Qur)::US11021454, Compound Quercetin::US9180183, Quercetin::med.21724, Compound 4
SMILES Oc1cc(O)c2c(c1)oc(-c1ccc(O)c(O)c1)c(O)c2=O
InChI Key InChIKey=REFJWTPEDVJJIY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 304 hits for monomerid = 7460
Found 304 hits for monomerid = 7460
TargetAldo-keto reductase family 1 member B1 [K65Q](Bos taurus (Cattle))
Xiangtan University
Curated by ChEMBL
Xiangtan University
Curated by ChEMBL
Affinity DataIC50: 2.85E+3nMAssay Description:Inhibition of bovine lens aldose reductase using DL-glyceraldehyde as substrate assessed as NADPH oxidation measured for 10 mins by spectrophotometri...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMpH: 7.0 T: 2°CAssay Description:Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/ [gamma-32P] ATP. 32...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1 [99-307](Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory
Affinity DataIC50: 2.30E+4nMpH: 7.0 T: 2°CAssay Description:Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/ [gamma-32P] ATP. 32...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B(Marthasterias glacialis (starfish))
Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory
Affinity DataIC50: 7.50E+4nMpH: 7.0 T: 2°CAssay Description:Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/ [gamma-32P] ATP. 32...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMT: 2°CAssay Description:Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/ [gamma-32P] ATP. 32...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Mrc
Mrc
Affinity DataIC50: 3.80E+3nM Kd: 280nMpH: 7.2 T: 2°CAssay Description:Binding was detected as a change in the intrinsic tryptophan fluorescence of the PI3K upon the addition of inhibitor. The inhibitor was incubated wit...More data for this Ligand-Target Pair
Affinity DataIC50: 2.14E+4nMpH: 6.0 T: 2°CAssay Description:The assay was carried out at room temperature for 10 min with salivary alpha-amylase, starch, and test compounds. The reducing sugar was determined b...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMpH: 7.0 T: 2°CAssay Description:The 96-well flat-bottomed plates were coated with recombinant GST-BAD. After the plates were blocked, the reaction buffer containing test compound an...More data for this Ligand-Target Pair
TargetM18 aspartyl aminopeptidase(Plasmodium falciparum 3D7)
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 385nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center Affiliation: The Scripps Research Institute, TSRI Assay Provide...More data for this Ligand-Target Pair
TargetProcathepsin L(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: >5.96E+4nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center Affiliation: The Scripps Research Institute, TSRI Assay Provide...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Eberhard Karls University of Tuebingen
Eberhard Karls University of Tuebingen
Affinity DataIC50: 3.45E+3nMT: 2°CAssay Description:The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Homo sapiens (Human))
Eberhard Karls University of Tuebingen
Eberhard Karls University of Tuebingen
Affinity DataIC50: 2.31E+3nMT: 2°CAssay Description:The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
Swami Ramanand Teerth Marathwada University
Swami Ramanand Teerth Marathwada University
Affinity DataIC50: 3.12E+3nMpH: 6.2 T: 2°CAssay Description:The reaction mixture contained 1 mL of 1 M potassium phosphate buffer (pH 6.2), 0.4 mM lithium sulfate and 5 µM 2-mercaptoethanol, 10 µM DL...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:H-DXR was pre-incubated during 2 min in the presence of the inhibitors at different concentrations and DXP (480 ÁM). NADPH (160 ÁM final concentratio...More data for this Ligand-Target Pair
Affinity DataIC50: 3.96E+3nMAssay Description:The kinase assay was performed using the EMD Millipore KinaseProfiler service assay protocol. Aurora B kinase was supplied by EMD Millipore Corp. The...More data for this Ligand-Target Pair
Affinity DataIC50: 3.50E+3nMAssay Description:The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w...More data for this Ligand-Target Pair
Affinity DataIC50: 3.50E+3nMT: 2°CAssay Description:The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.53E+4nMpH: 7.4 T: 2°CAssay Description:Fluorescence intensity was measured at 420 nm excitation and 485 nm emission using a microplate reader (MPR-A4╬╣II; TOSOH, Tokyo, Japan, or Fluoroska...More data for this Ligand-Target Pair
Affinity DataIC50: 7.41E+4nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Catholic University of Daegu
Catholic University of Daegu
Affinity DataIC50: 4.09E+4nMpH: 6.0Assay Description:PTP1B activity was measured by adding 2mM p-NPP and PTP1B in a 50 mM citrate buffer (pH 6.0, 0.1 M NaCl, 1 mMEDTA, and 1 mM dithiothreitol), with or ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataIC50: 7.28E+4nMpH: 8.0 T: 2°CAssay Description:Briefly, 140 μL of sodium phosphate buffer (pH 8.0), 20 μL of each tested compound with different concentrations (4, 20, and 100 μM) a...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMpH: 8.0Assay Description:The rate of hydrolysis of the substrates (0.3 mM) by trypsin (18 U/ml), thrombin (12.5 NIH U/ml), urokinase (62500 IU/ml) and elastase (8.4 U/ml) was...More data for this Ligand-Target Pair
Affinity DataIC50: 3.50E+4nMpH: 8.0Assay Description:The rate of hydrolysis of the substrates (0.3 mM) by trypsin (18 U/ml), thrombin (12.5 NIH U/ml), urokinase (62500 IU/ml) and elastase (8.4 U/ml) was...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMpH: 8.0Assay Description:The rate of hydrolysis of the substrates (0.3 mM) by trypsin (18 U/ml), thrombin (12.5 NIH U/ml), urokinase (62500 IU/ml) and elastase (8.4 U/ml) was...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMpH: 8.0Assay Description:The rate of hydrolysis of the substrates (0.3 mM) by trypsin (18 U/ml), thrombin (12.5 NIH U/ml), urokinase (62500 IU/ml) and elastase (8.4 U/ml) was...More data for this Ligand-Target Pair
TargetInterstitial collagenase [100-268](Homo sapiens (Human))
East China University of Science and Technology
East China University of Science and Technology
Affinity DataIC50: 1.49E+3nMpH: 7.5Assay Description:The activity of cd-MMP-1 was measured using a fluorescence-based assay. It was performed in white 96-well half area microplate (Greiner) in a final v...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of PIM1 kinaseChecked by AuthorMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Rattus norvegicus)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:In vitro inhibition of 5-lipoxygenase (5-lo) from the 20000 g supernatant of RBI-1 cellsMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Rattus norvegicus)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:In vitro inhibition of 5-lipoxygenase in rat (peritoneal assay)More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Ability to inhibit protein-tyrosine kinase activity of p56lck (isolated from bovine thymus) in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 2.36E+4nMAssay Description:IC50 was measured as concentration required to inhibit 50% of HIV-integrase cleavageMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1 [K65Q](Bos taurus (Cattle))
Xiangtan University
Curated by ChEMBL
Xiangtan University
Curated by ChEMBL
Affinity DataIC50: 1.03E+4nMAssay Description:Inhibition of Aldehyde reductase 2More data for this Ligand-Target Pair
Affinity DataIC50: 7.10E+3nMAssay Description:Inhibitory concentration of the compounds against Bovine trypsin enzyme.More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1 [K65Q](Bos taurus (Cattle))
Xiangtan University
Curated by ChEMBL
Xiangtan University
Curated by ChEMBL
Affinity DataIC50: 3.29E+4nMAssay Description:Inhibition of ALR2 (aldose reductase) of bovine lensMore data for this Ligand-Target Pair
Affinity DataIC50: 1.21E+4nMAssay Description:Inhibition of urokinase amidolytic activity (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of thrombin (unknown origin) assessed as hydrolysis of N-benzoyl-phenylalanylvalyl-arginine-paranitroanilideMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of human thrombin amidolytic activity using D-Phe-Pip-Arg-pNA as substrate preincubated for 10 mins followed by substrate addition measure...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Rattus norvegicus)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:In vitro inhibition against 5-lipoxygenase in RBL-1 cells was determinedMore data for this Ligand-Target Pair
Affinity DataIC50: 43nMAssay Description:Inhibitory activity against PIM1More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 3.35E+4nMAssay Description:Inhibition of sEH (unknown origin) assessed as substrate PHOME hydrolysis after 1 hr by fluorescence methodMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of recombinant HIV-1 integrase 3'-processing activity using 32P 5' end-labeled linear 21'mer as substrate preincubated for 30 mins prior t...More data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of recombinant HIV-1 integrase strand transfer activity using 32P 5' end-labeled linear 21'mer as substrate preincubated for 30 mins prior...More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of His6-tagged HIV-1 integrase assessed as decrease in integrase-Flag-LEDGF/p75 interaction preincubated with enzyme for 30 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 8.32E+3nMAssay Description:In vitro inhibitory activity against aldose reductase (ALR2) from rat lens extraction.More data for this Ligand-Target Pair
Affinity DataIC50: 3.56E+4nMAssay Description:In vitro inhibitory activity against sorbitol dehydrogenase (SD) from sheep liverMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Homo sapiens (Human))
Universidad De Santiago De Chile
Curated by ChEMBL
Universidad De Santiago De Chile
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of 15-hLO2More data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(Human SARS coronavirus (SARS-CoV) (Severe acute re...)
Konkuk University
Curated by ChEMBL
Konkuk University
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of ATPase activity of SARS coronavirus helicase assessed as phosphate release by malachite green assayMore data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(Human SARS coronavirus (SARS-CoV) (Severe acute re...)
Konkuk University
Curated by ChEMBL
Konkuk University
Curated by ChEMBL
Affinity DataIC50: 8.10E+3nMAssay Description:Inhibition of SARS coronavirus helicase assessed as duplex-DNA unwinding by FRET based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.32E+5nMAssay Description:Inhibition of p56 lckMore data for this Ligand-Target Pair
Activity Spreadsheet -- ITC Data from BindingDB
 Found 4 hits for monomerid = 7460
Found 4 hits for monomerid = 7460
ITC DataΔG°: -9.78kcal/mole −TΔS°: -0.257kcal/mole ΔH°: -9.58kcal/mole logk: 3.93E+7
pH: 7.5 T: 10.00°C
pH: 7.5 T: 10.00°C
ITC DataΔG°: -8.08kcal/mole −TΔS°: 0.840kcal/mole ΔH°: -8.92kcal/mole logk: 6.82E+5
pH: 7.0 T: 30.00°C
pH: 7.0 T: 30.00°C
ITC DataΔG°: -7.40kcal/mole −TΔS°: -2.66kcal/mole ΔH°: -4.74kcal/mole logk: 2.20E+5
pH: 7.0 T: 30.00°C
pH: 7.0 T: 30.00°C
ITC DataΔG°: -7.94kcal/mole −TΔS°: -2.82kcal/mole ΔH°: -5.12kcal/mole logk: 5.42E+5
pH: 7.0 T: 30.00°C
pH: 7.0 T: 30.00°C
 3D Structure (crystal)
3D Structure (crystal)