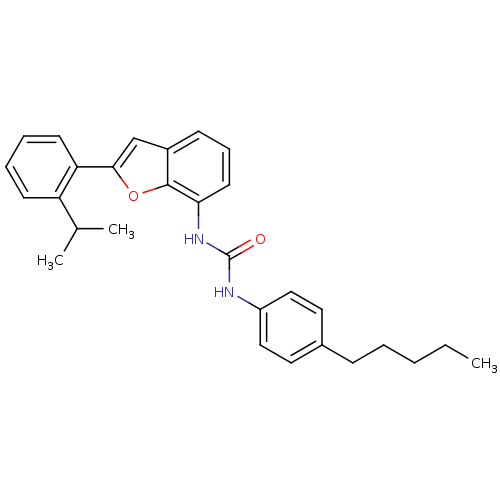
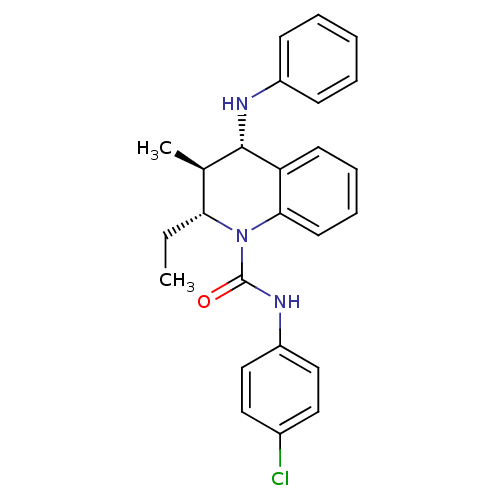
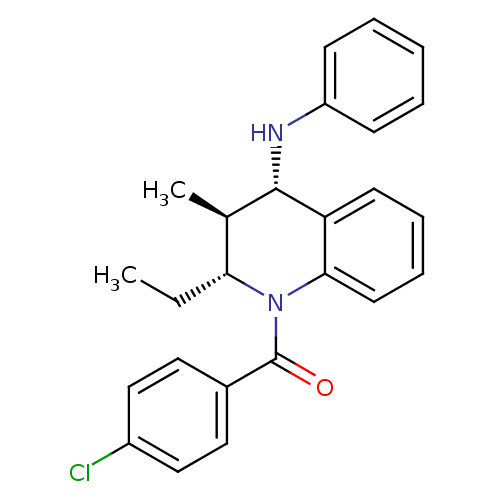
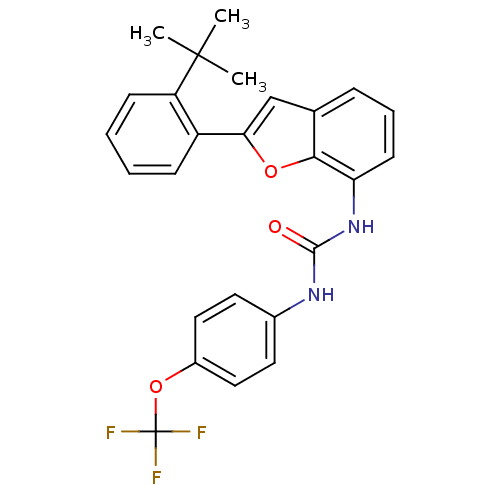
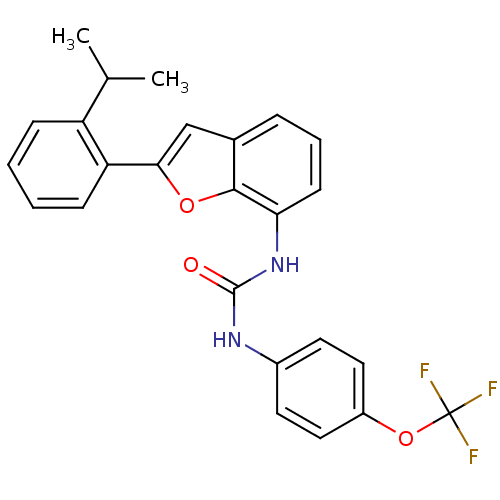
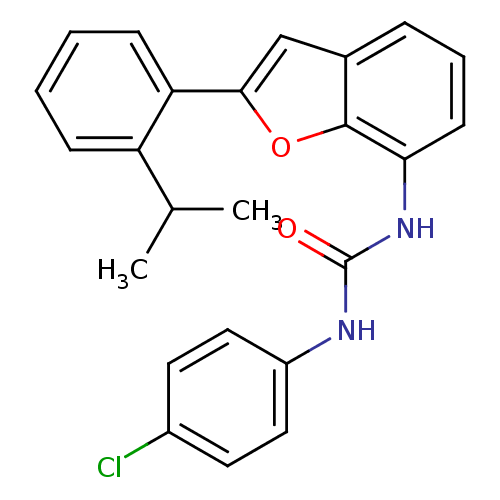
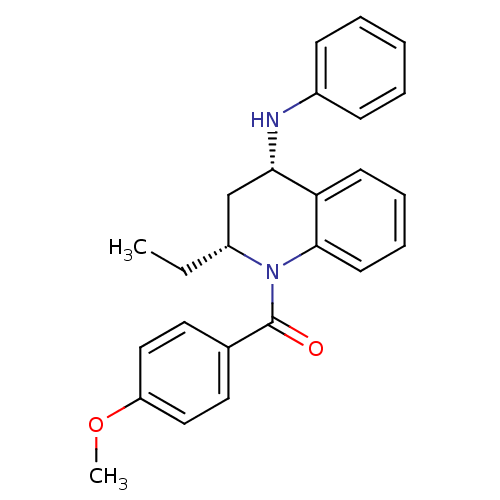
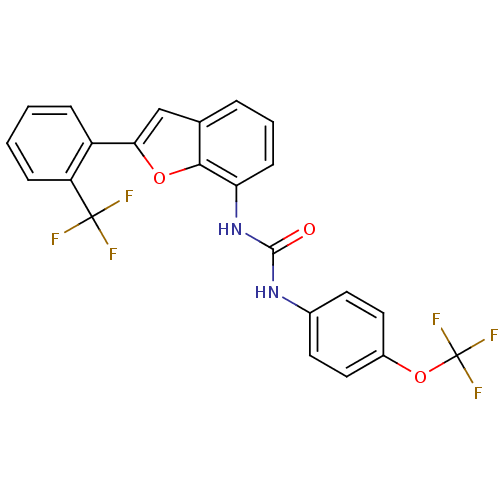
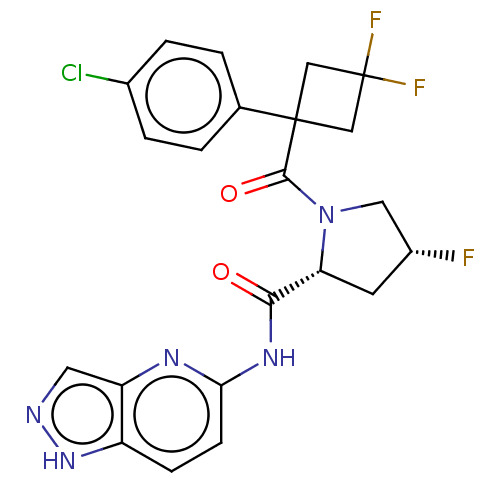
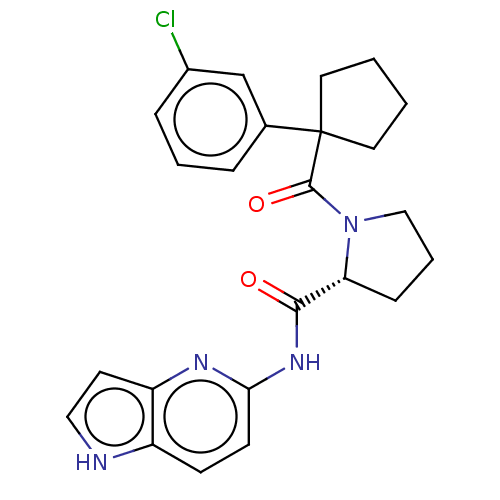
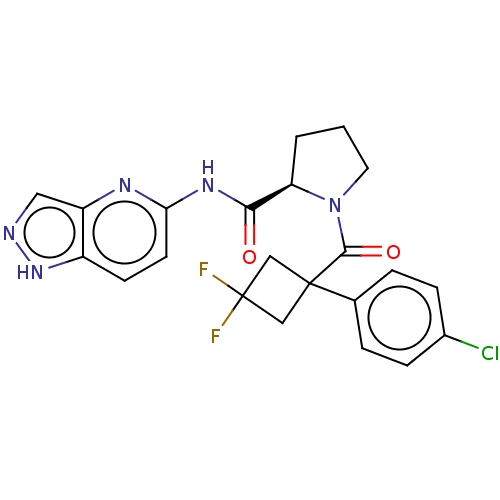
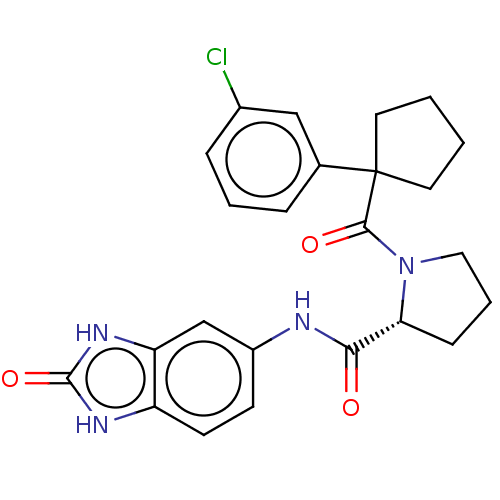
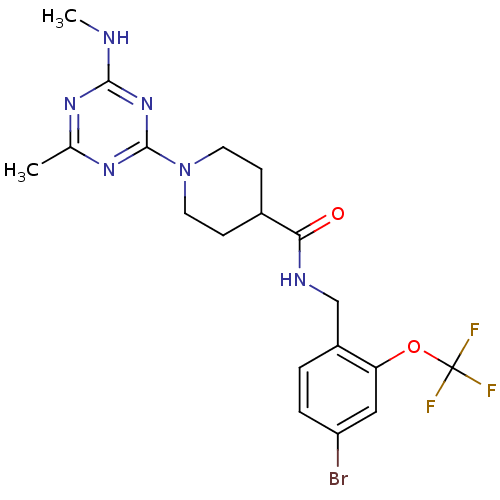
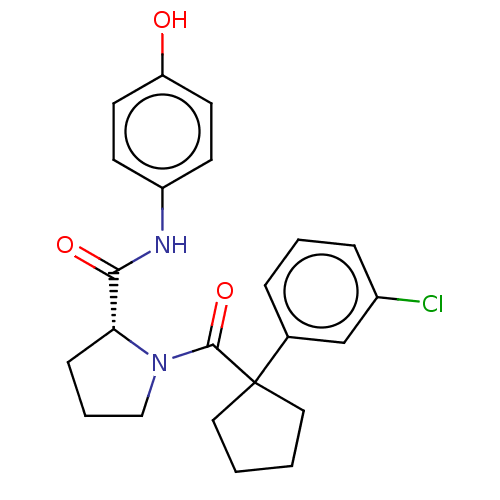
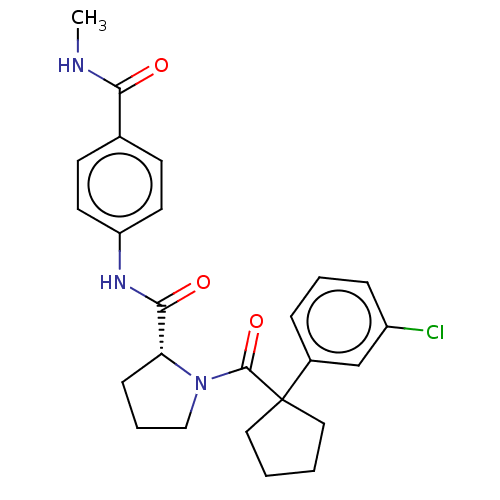
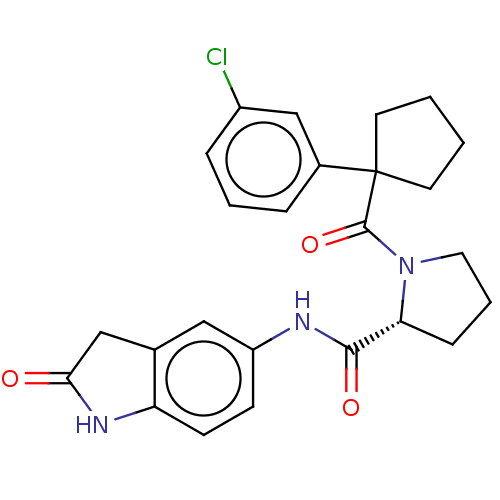
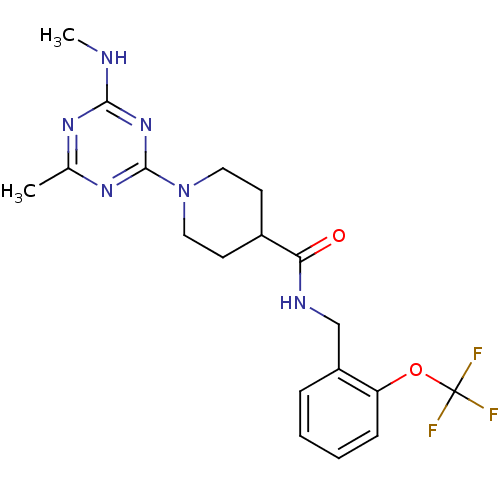
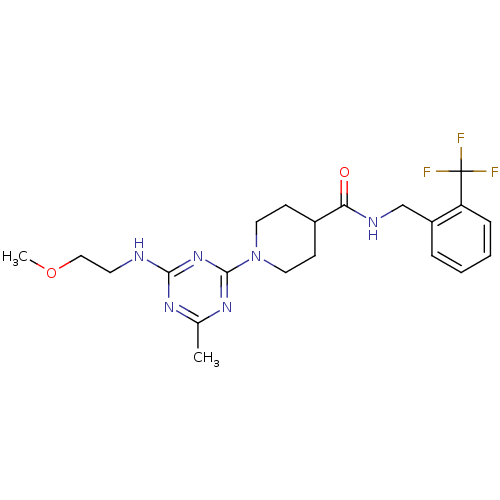
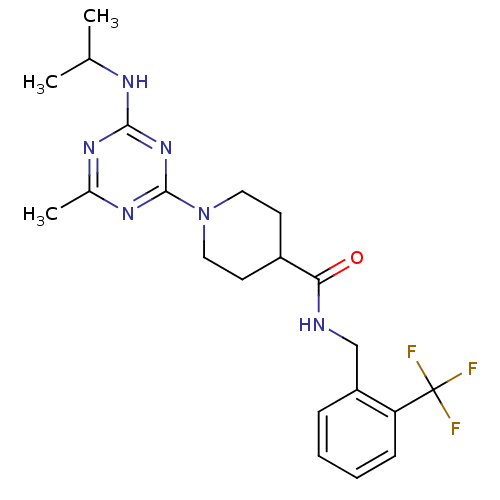
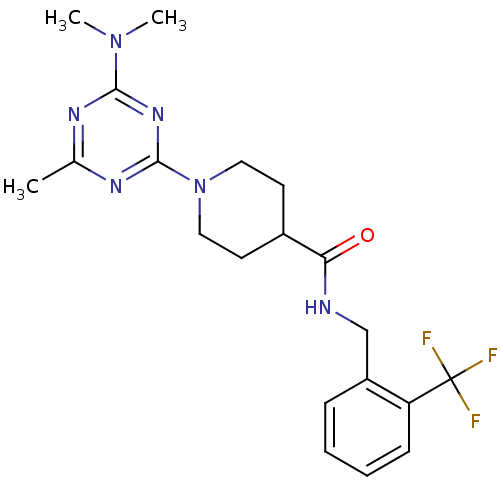
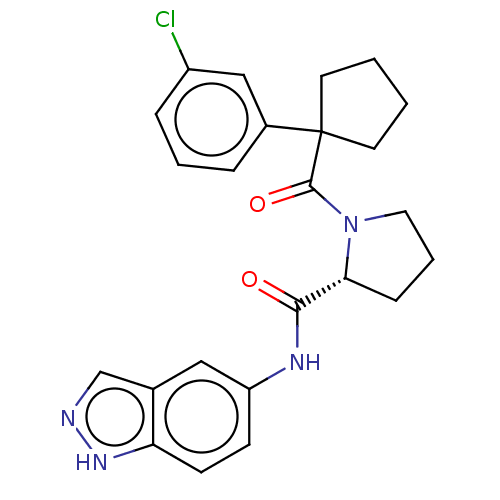
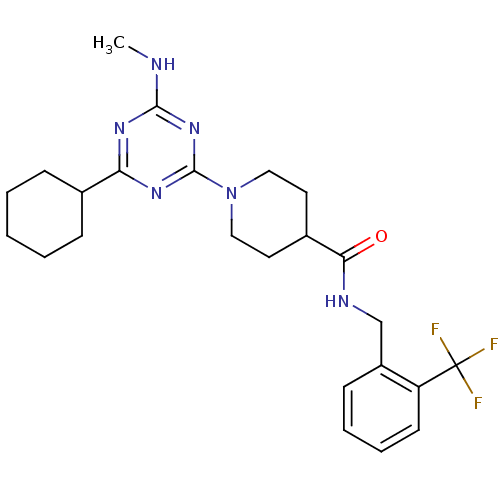
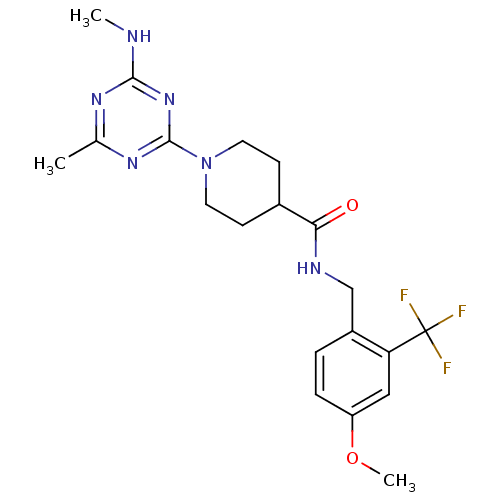
Affinity DataKi: 70nMAssay Description:Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cellsMore data for this Ligand-Target Pair
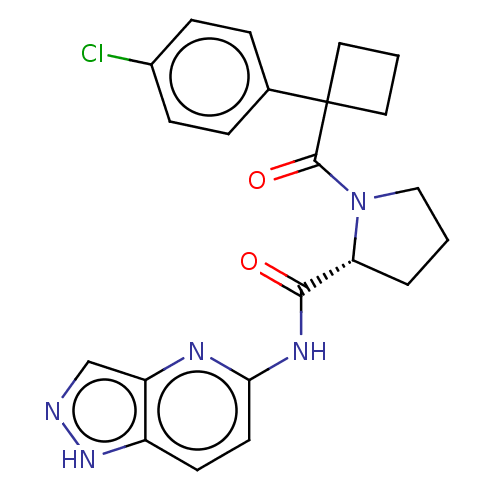
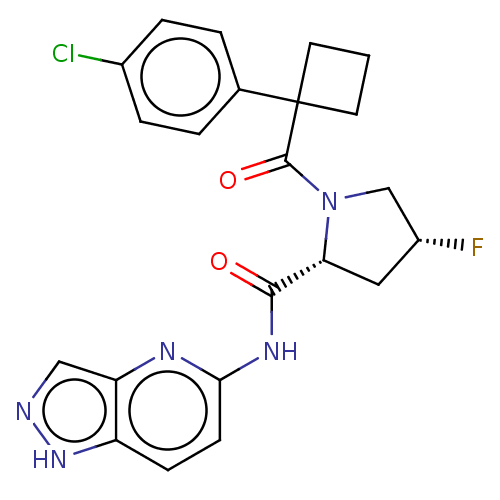
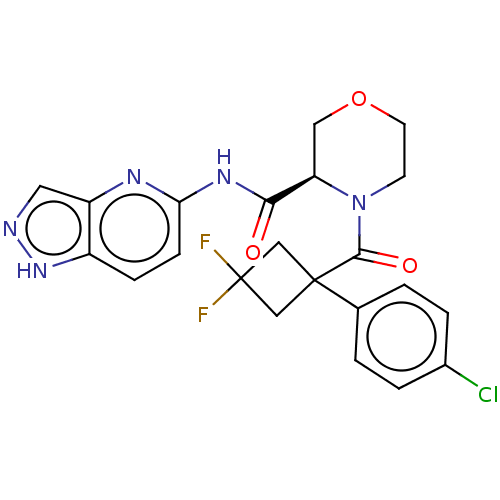
Affinity DataKi: 100nMAssay Description:Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cellsMore data for this Ligand-Target Pair
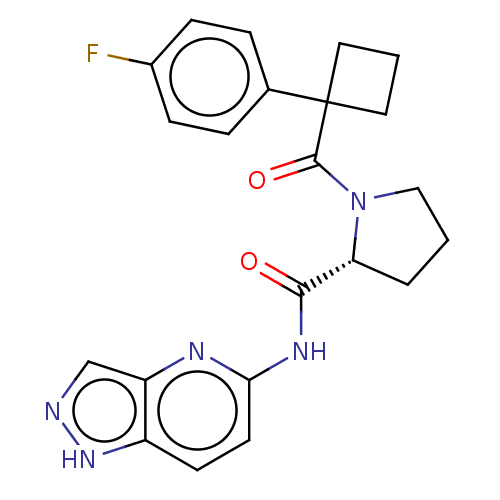
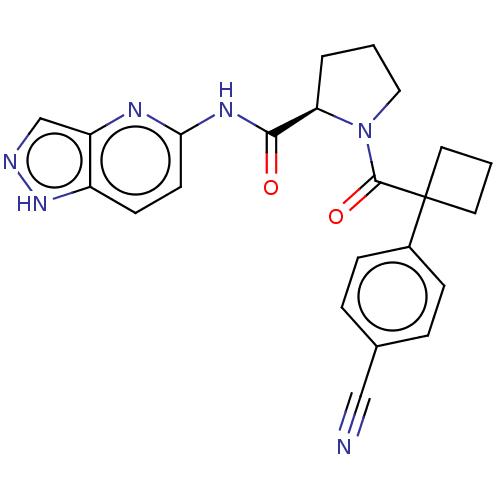
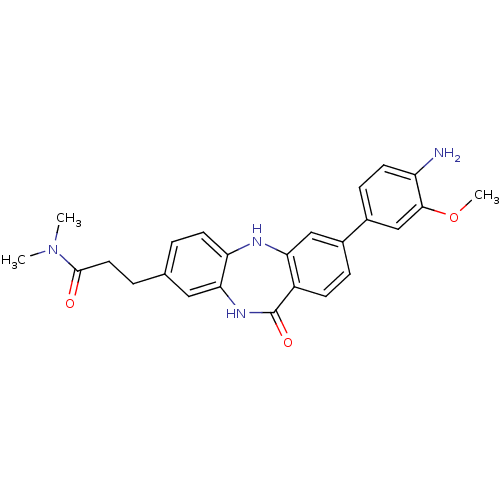
Affinity DataKi: 100nMAssay Description:Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cellsMore data for this Ligand-Target Pair
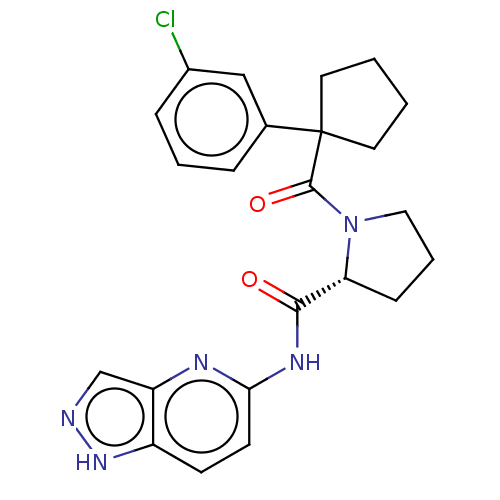
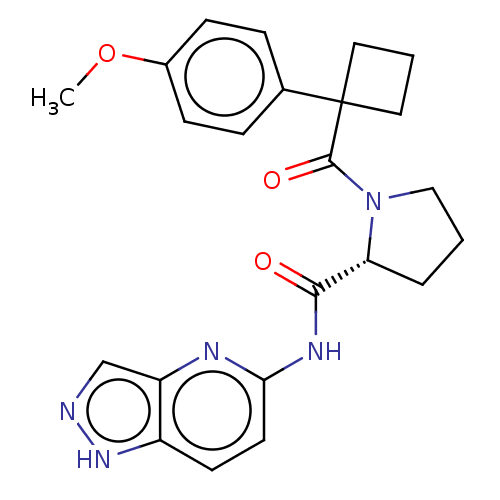
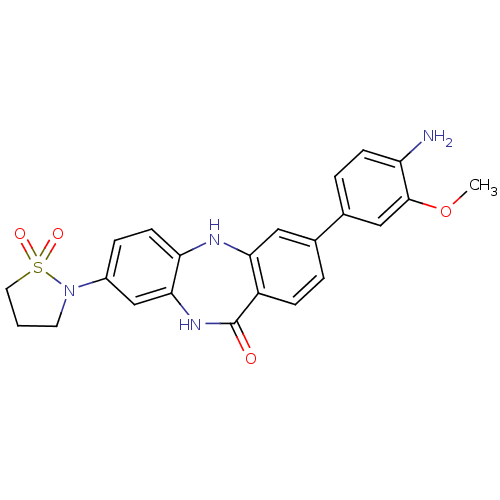
Affinity DataKi: 100nMAssay Description:Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cellsMore data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:Displacement of [33P]2-MeS-ADP from human P2Y1 receptor expressed in human U2OS cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cellsMore data for this Ligand-Target Pair
Affinity DataKi: 300nMAssay Description:Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cellsMore data for this Ligand-Target Pair
Affinity DataKi: 300nMAssay Description:Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cellsMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cellsMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cellsMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cellsMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Displacement of [33P]2-MeS-ADP from human P2Y1 receptor expressed in human U2OS cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 500nMAssay Description:Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cellsMore data for this Ligand-Target Pair
Affinity DataKi: 500nMAssay Description:Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cellsMore data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Displacement of [33P]2-MeS-ADP from human P2Y1 receptor expressed in human U2OS cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 640nMAssay Description:Displacement of [33P]2-MeS-ADP from human P2Y1 receptor expressed in human U2OS cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 760nMAssay Description:Displacement of [33P]2-MeS-ADP from human P2Y1 receptor expressed in human U2OS cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 800nMAssay Description:Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.50E+3nMAssay Description:Displacement of [33P]2-MeS-ADP from human P2Y1 receptor expressed in human U2OS cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0794nMAssay Description:Inhibition of full-length P300 (unknown origin) using ARTKQTARKSTGGKAPRKQLAGG-K(Biotin)-amide as substrate preincubated for 60 mins followed by subst...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of full-length P300 (unknown origin) using ARTKQTARKSTGGKAPRKQLAGG-K(Biotin)-amide as substrate preincubated for 60 mins followed by subst...More data for this Ligand-Target Pair
Affinity DataIC50: 0.126nMAssay Description:Inhibition of full-length P300 (unknown origin) using ARTKQTARKSTGGKAPRKQLAGG-K(Biotin)-amide as substrate preincubated for 60 mins followed by subst...More data for this Ligand-Target Pair
Affinity DataIC50: 0.158nMAssay Description:Inhibition of full-length P300 (unknown origin) using ARTKQTARKSTGGKAPRKQLAGG-K(Biotin)-amide as substrate preincubated for 60 mins followed by subst...More data for this Ligand-Target Pair
Affinity DataIC50: 0.158nMAssay Description:Inhibition of full-length P300 (unknown origin) using ARTKQTARKSTGGKAPRKQLAGG-K(Biotin)-amide as substrate preincubated for 60 mins followed by subst...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of full-length P300 (unknown origin) using ARTKQTARKSTGGKAPRKQLAGG-K(Biotin)-amide as substrate preincubated for 60 mins followed by subst...More data for this Ligand-Target Pair
Affinity DataIC50: 0.251nMAssay Description:Inhibition of full-length P300 (unknown origin) using ARTKQTARKSTGGKAPRKQLAGG-K(Biotin)-amide as substrate preincubated for 60 mins followed by subst...More data for this Ligand-Target Pair
Affinity DataIC50: 0.251nMAssay Description:Inhibition of full-length P300 (unknown origin) using ARTKQTARKSTGGKAPRKQLAGG-K(Biotin)-amide as substrate preincubated for 60 mins followed by subst...More data for this Ligand-Target Pair
Affinity DataIC50: 0.316nMAssay Description:Inhibition of full-length P300 (unknown origin) using ARTKQTARKSTGGKAPRKQLAGG-K(Biotin)-amide as substrate preincubated for 60 mins followed by subst...More data for this Ligand-Target Pair
Affinity DataIC50: 0.398nMAssay Description:Inhibition of full-length P300 (unknown origin) using ARTKQTARKSTGGKAPRKQLAGG-K(Biotin)-amide as substrate preincubated for 60 mins followed by subst...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 0.631nMAssay Description:Inhibition of full-length P300 (unknown origin) using ARTKQTARKSTGGKAPRKQLAGG-K(Biotin)-amide as substrate preincubated for 60 mins followed by subst...More data for this Ligand-Target Pair
Affinity DataIC50: 0.631nMAssay Description:Inhibition of full-length P300 (1 to 2414 residues) (unknown origin) using ARTKQTARKSTGGKAPRKQLAGG-K(Biotin)-amide as substrate preincubated for 60 m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.794nMAssay Description:Inhibition of full-length P300 (unknown origin) using ARTKQTARKSTGGKAPRKQLAGG-K(Biotin)-amide as substrate preincubated for 60 mins followed by subst...More data for this Ligand-Target Pair
Affinity DataIC50: 0.794nMAssay Description:Inhibition of full-length P300 (unknown origin) using ARTKQTARKSTGGKAPRKQLAGG-K(Biotin)-amide as substrate preincubated for 60 mins followed by subst...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of full-length P300 (unknown origin) using ARTKQTARKSTGGKAPRKQLAGG-K(Biotin)-amide as substrate preincubated for 60 mins followed by subst...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of full-length P300 (1 to 2414 residues) (unknown origin) using ARTKQTARKSTGGKAPRKQLAGG-K(Biotin)-amide as substrate preincubated for 60 m...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of full-length P300 (unknown origin) using ARTKQTARKSTGGKAPRKQLAGG-K(Biotin)-amide as substrate preincubated for 60 mins followed by subst...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human recombinant Chk1More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human recombinant Chk1More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of rat soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prior ...More data for this Ligand-Target Pair