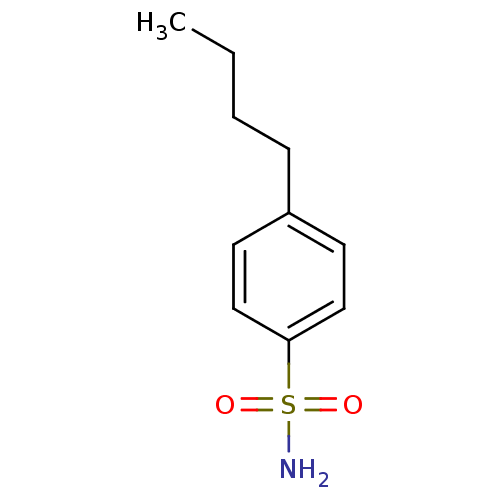
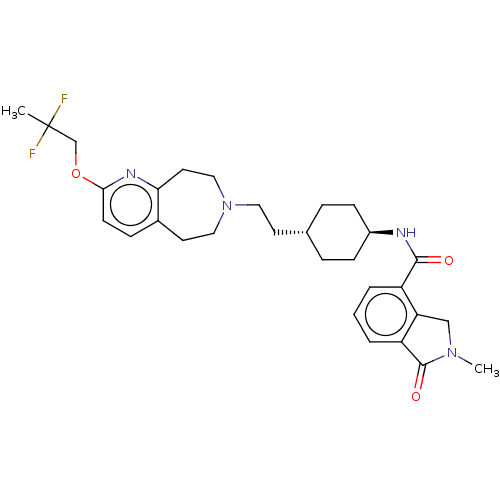
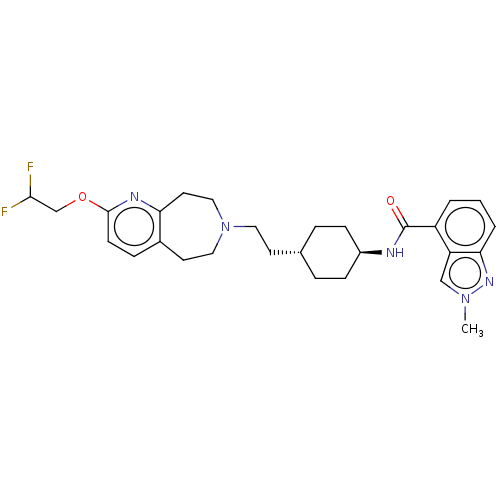
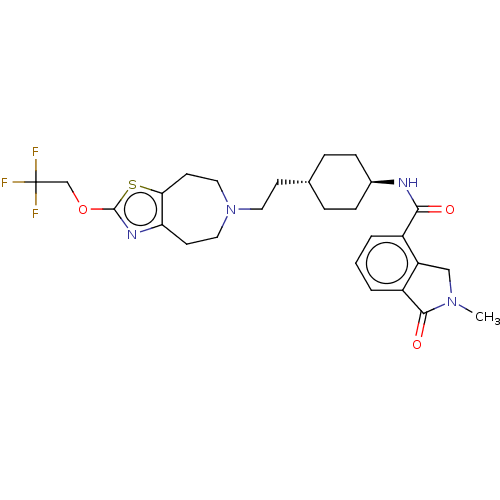
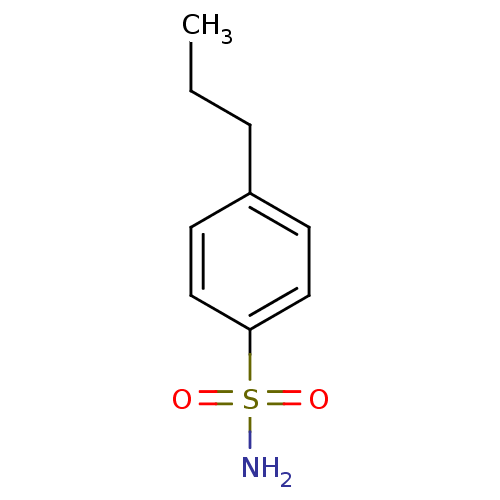
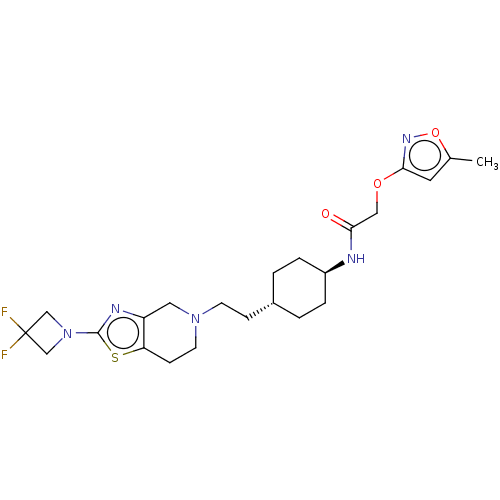
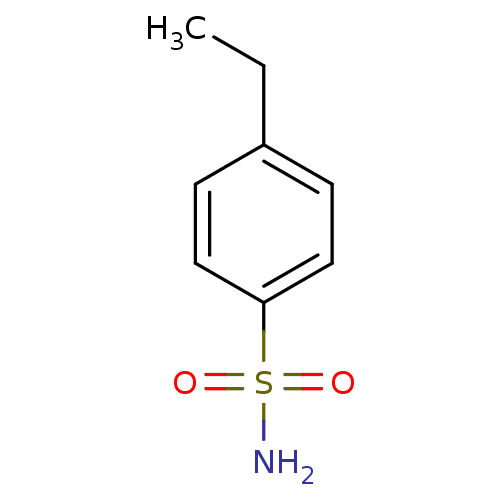
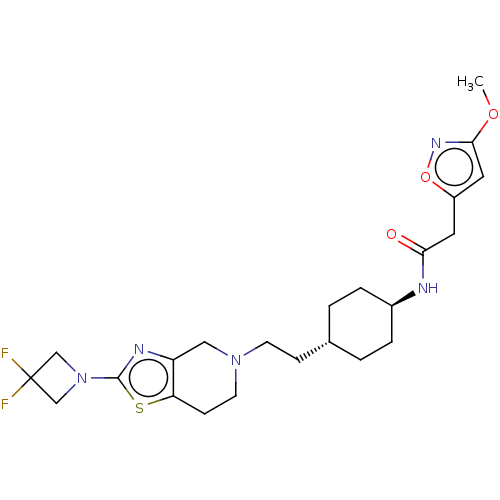
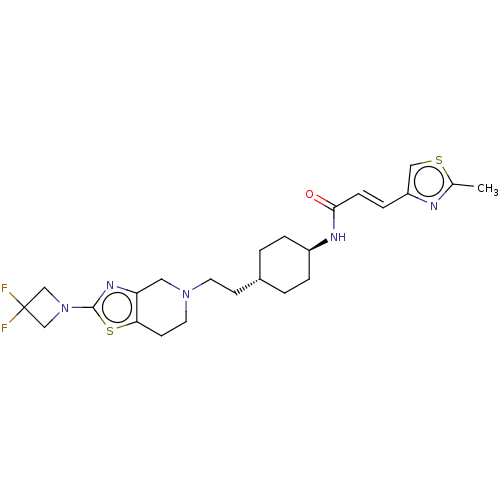
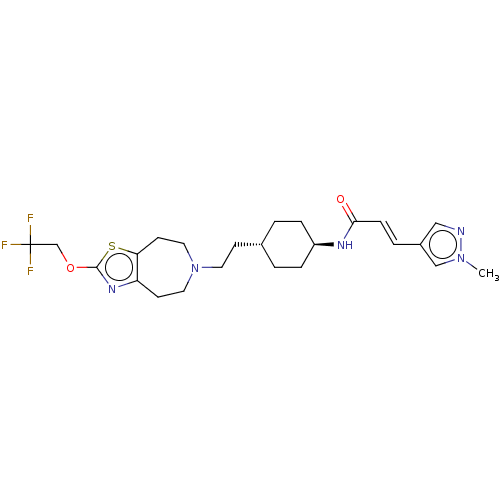
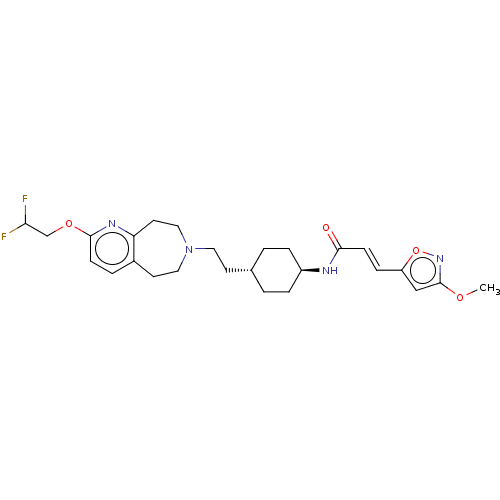
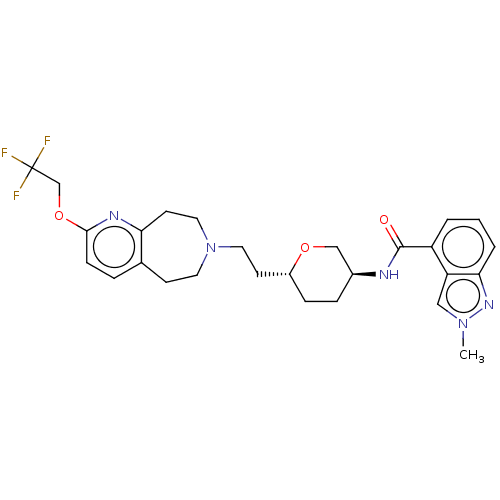
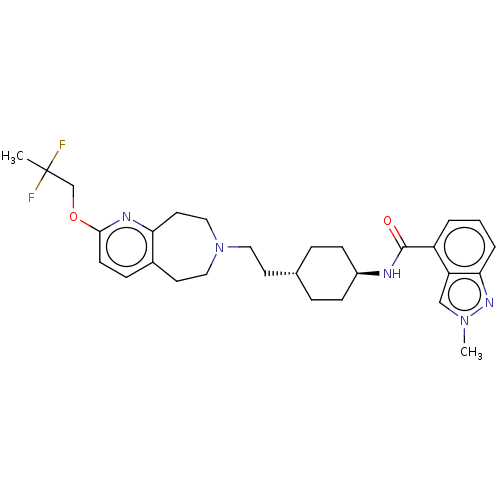
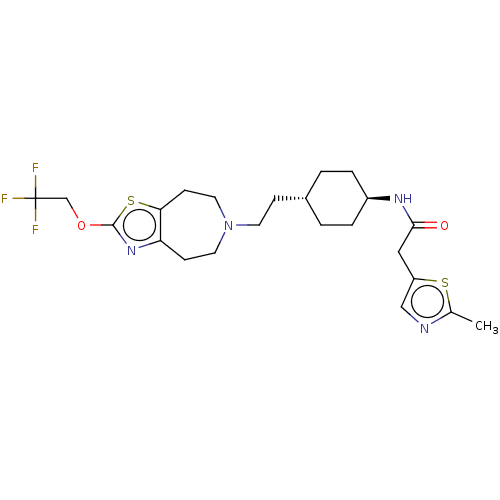
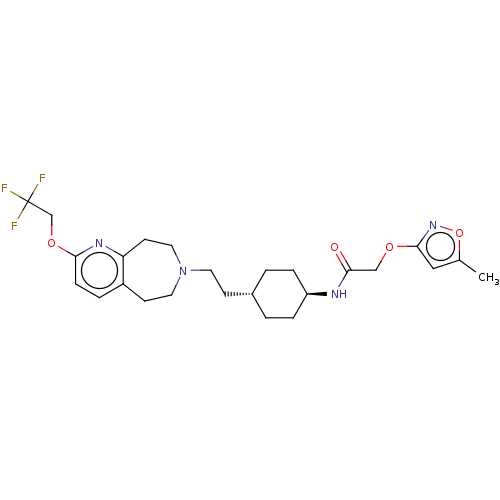
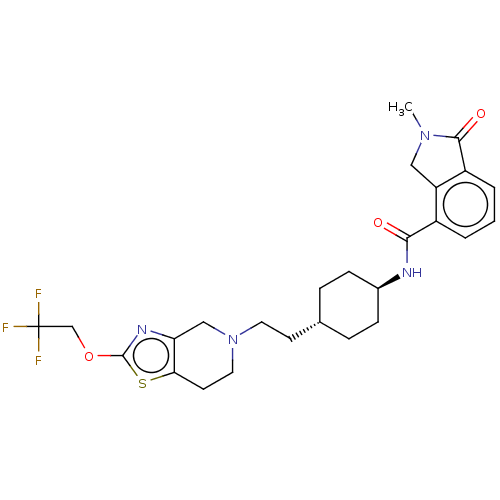
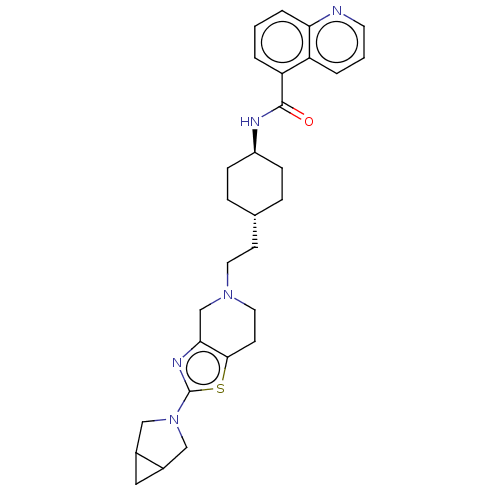
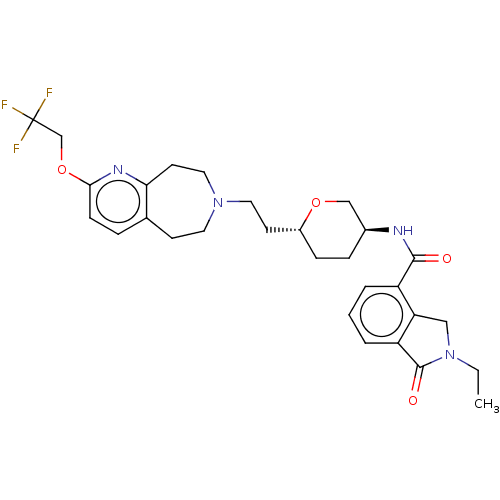
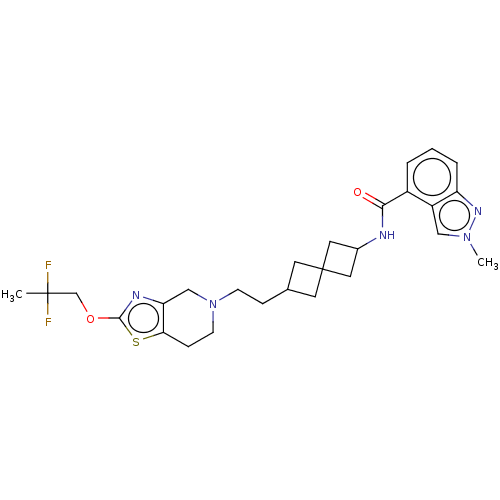
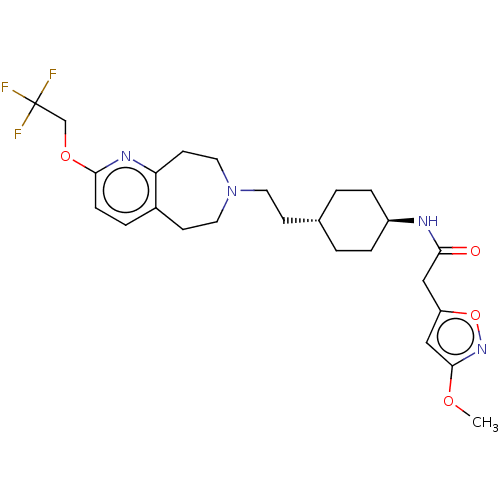
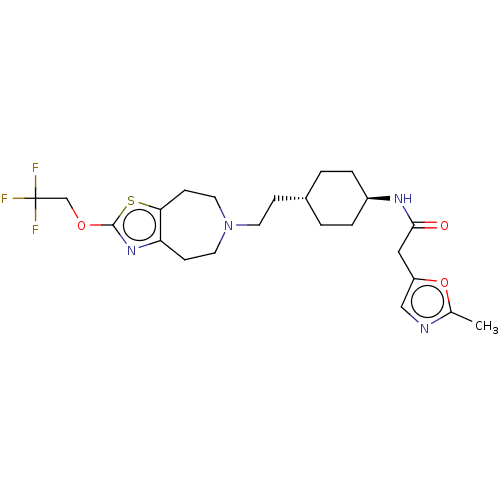
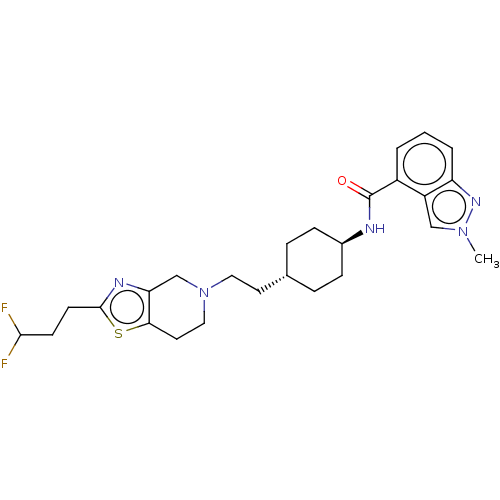
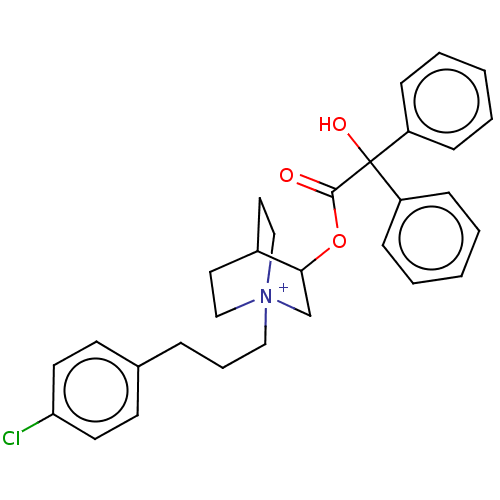
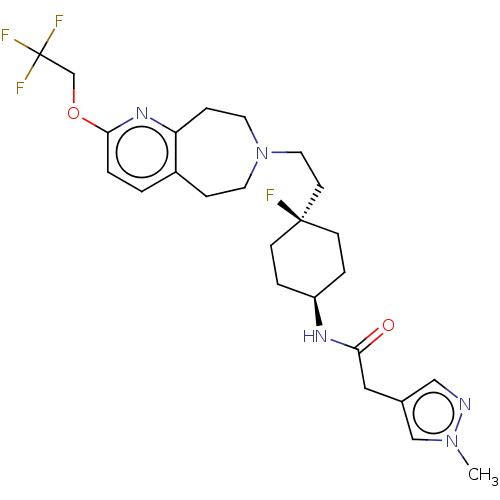
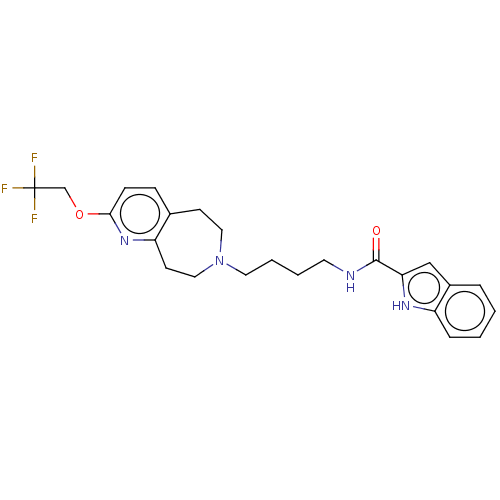
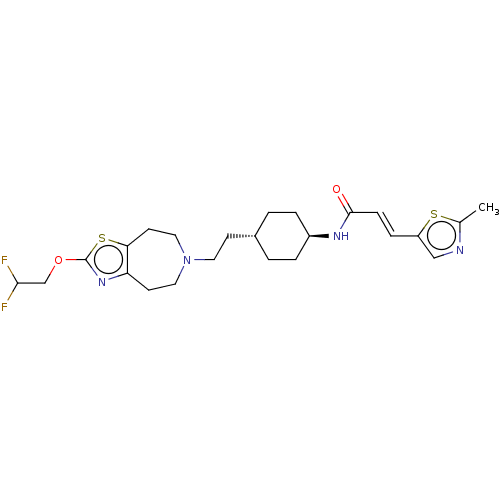
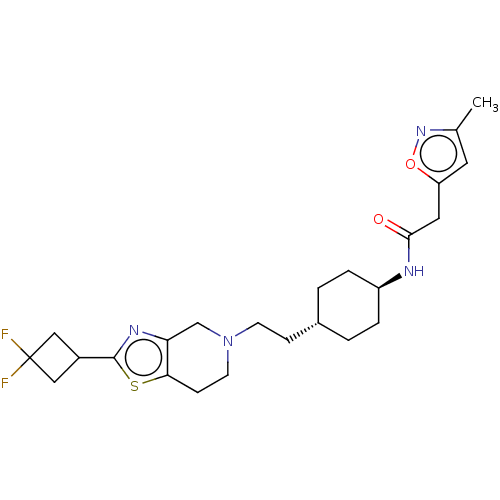
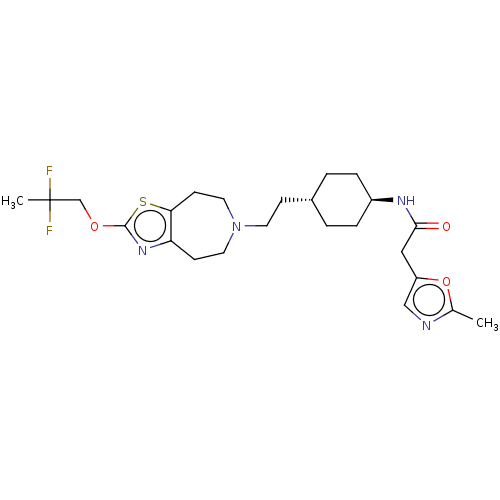
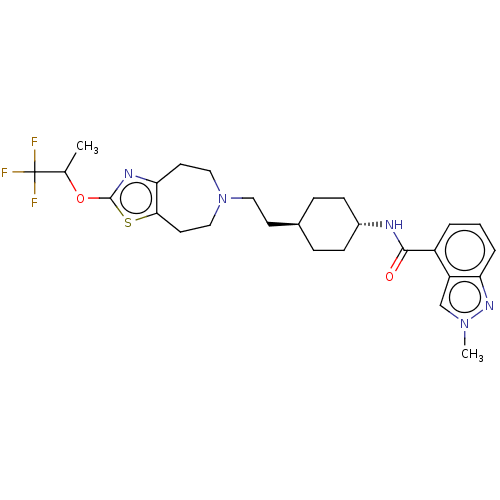
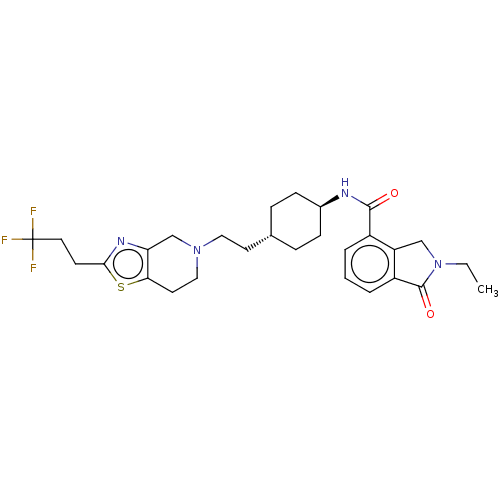
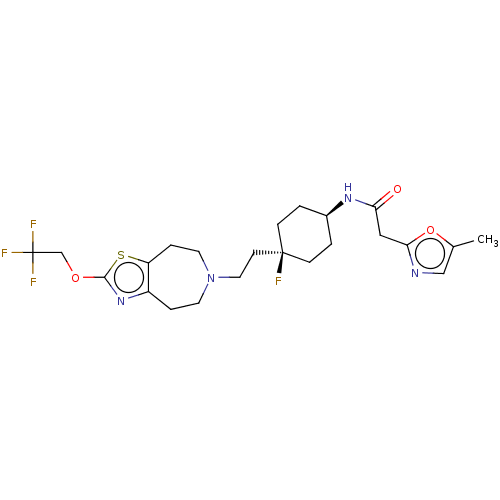
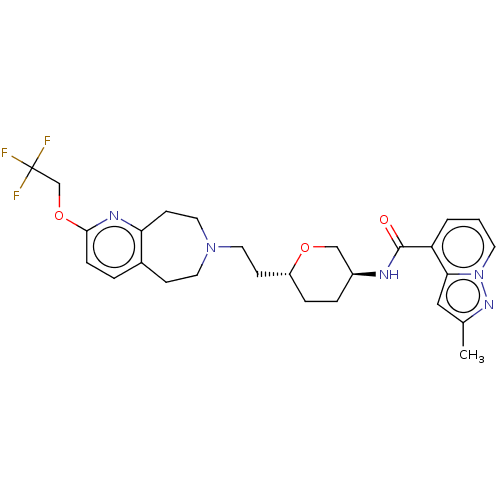
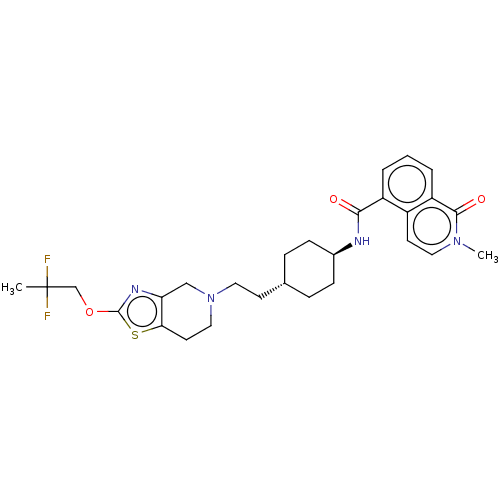
TargetCarbonic anhydrase 2(Homo sapiens (Human))
The University Of Tokushima Graduate School
Curated by ChEMBL
The University Of Tokushima Graduate School
Curated by ChEMBL
Affinity DataKi: 0.000800nMAssay Description:Binding affinity to human carbonic anhydrase 2More data for this Ligand-Target Pair
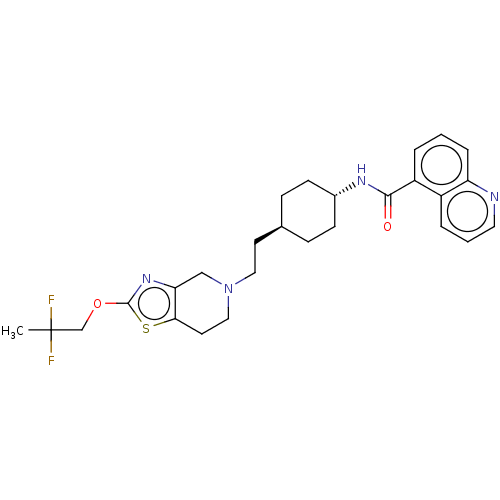
TargetCarbonic anhydrase 2(Homo sapiens (Human))
The University Of Tokushima Graduate School
Curated by ChEMBL
The University Of Tokushima Graduate School
Curated by ChEMBL
Affinity DataKi: 0.000800nMAssay Description:Binding affinity to human carbonic anhydrase 2More data for this Ligand-Target Pair
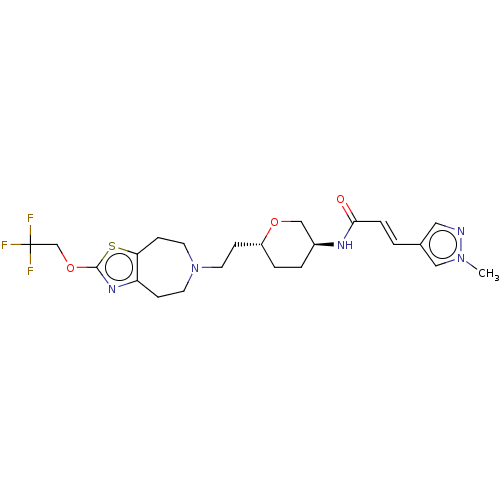
TargetCarbonic anhydrase 2(Homo sapiens (Human))
The University Of Tokushima Graduate School
Curated by ChEMBL
The University Of Tokushima Graduate School
Curated by ChEMBL
Affinity DataKi: 0.00500nMAssay Description:Binding affinity to human carbonic anhydrase 2More data for this Ligand-Target Pair
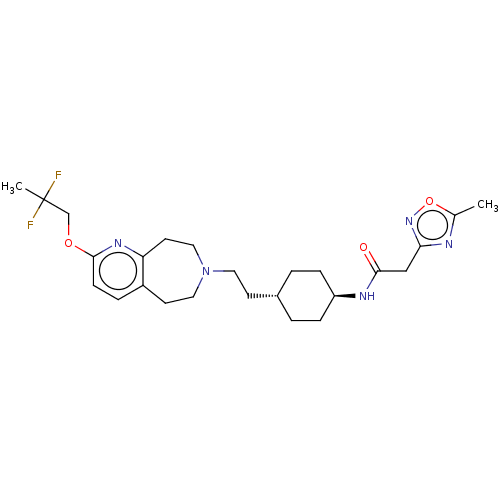
TargetCarbonic anhydrase 2(Homo sapiens (Human))
The University Of Tokushima Graduate School
Curated by ChEMBL
The University Of Tokushima Graduate School
Curated by ChEMBL
Affinity DataKi: 0.00500nMAssay Description:Binding affinity to human carbonic anhydrase 2More data for this Ligand-Target Pair
Affinity DataKi: 0.0120nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0140nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0220nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0260nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 2(Homo sapiens (Human))
The University Of Tokushima Graduate School
Curated by ChEMBL
The University Of Tokushima Graduate School
Curated by ChEMBL
Affinity DataKi: 0.0300nMAssay Description:Binding affinity to human carbonic anhydrase 2More data for this Ligand-Target Pair
TargetCarbonic anhydrase 2(Homo sapiens (Human))
The University Of Tokushima Graduate School
Curated by ChEMBL
The University Of Tokushima Graduate School
Curated by ChEMBL
Affinity DataKi: 0.0300nMAssay Description:Binding affinity to human carbonic anhydrase 2More data for this Ligand-Target Pair
Affinity DataKi: 0.0350nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0480nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 2(Homo sapiens (Human))
The University Of Tokushima Graduate School
Curated by ChEMBL
The University Of Tokushima Graduate School
Curated by ChEMBL
Affinity DataKi: 0.0500nMAssay Description:Binding affinity to human carbonic anhydrase 2More data for this Ligand-Target Pair
TargetCarbonic anhydrase 2(Homo sapiens (Human))
The University Of Tokushima Graduate School
Curated by ChEMBL
The University Of Tokushima Graduate School
Curated by ChEMBL
Affinity DataKi: 0.0500nMAssay Description:Binding affinity to human carbonic anhydrase 2More data for this Ligand-Target Pair
Affinity DataKi: 0.0500nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0510nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0520nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0570nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0580nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0590nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0640nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0670nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0750nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0770nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0800nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0850nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0860nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0871nMAssay Description:Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 0.0920nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0950nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.0960nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
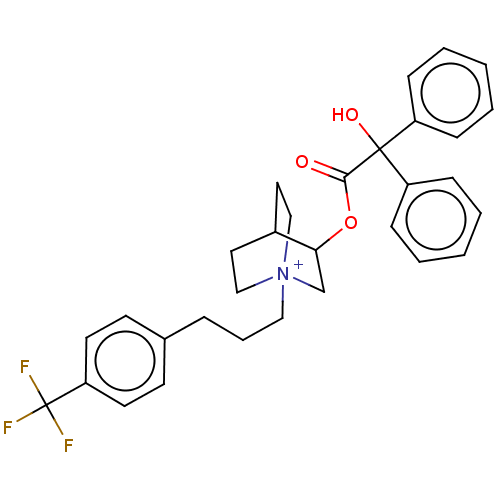
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
National Institute Of Advanced Industrial Science And Technology (Aist)
Curated by ChEMBL
National Institute Of Advanced Industrial Science And Technology (Aist)
Curated by ChEMBL
Affinity DataKi: 0.110nMAssay Description:Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.120nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.120nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.120nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.120nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.150nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.150nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent...More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
Kitasato University
Curated by ChEMBL
Kitasato University
Curated by ChEMBL
Affinity DataKi: 0.178nMAssay Description:Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cerebellum after 1 hr by liquid scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
National Institute Of Advanced Industrial Science And Technology (Aist)
Curated by ChEMBL
National Institute Of Advanced Industrial Science And Technology (Aist)
Curated by ChEMBL
Affinity DataKi: 0.180nMAssay Description:Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting methodMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)