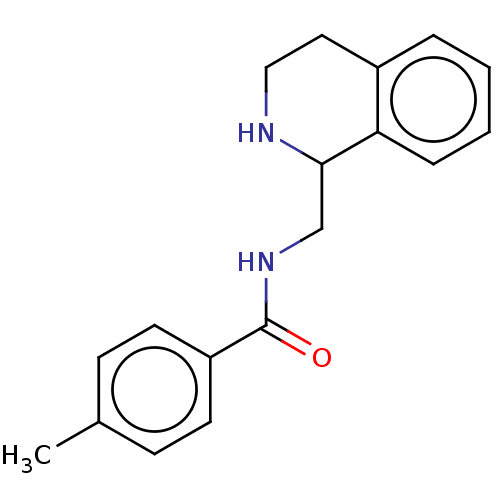
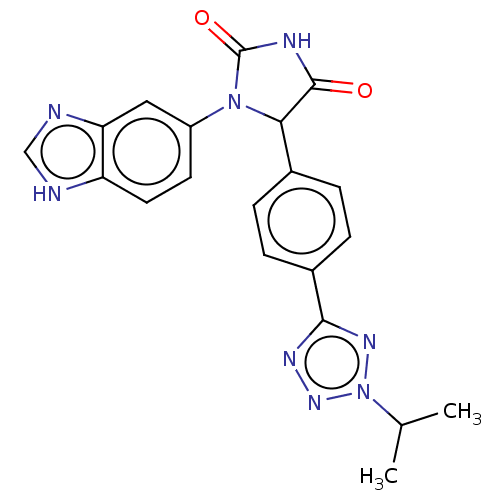
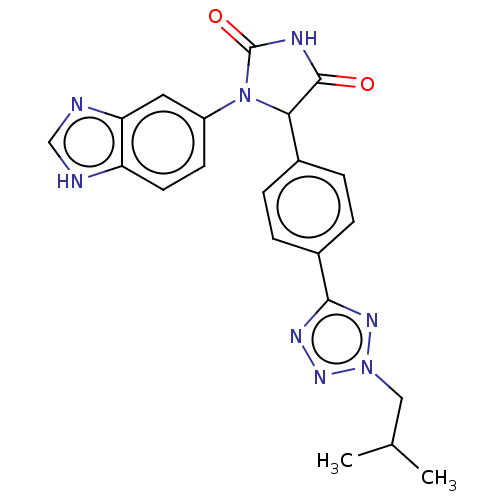
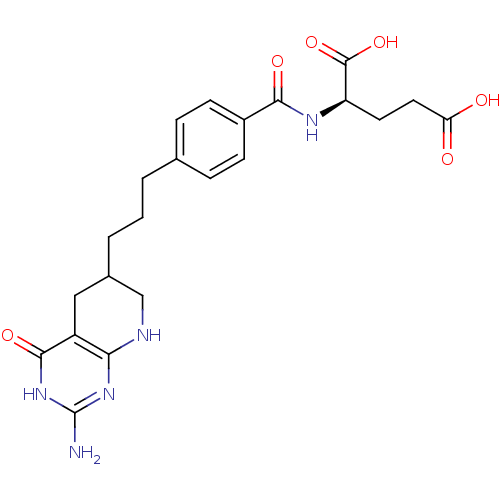
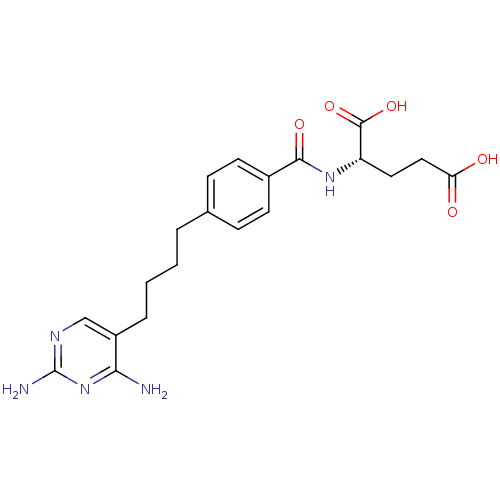
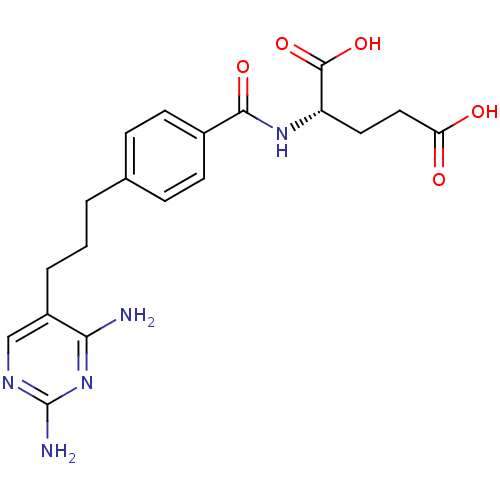
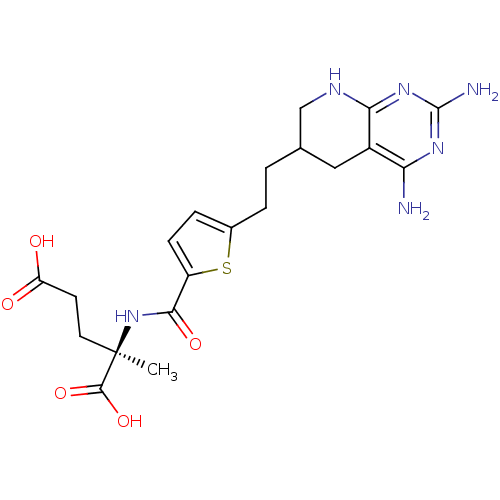
Affinity DataKi: 0.0000190nMAssay Description:Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cellsMore data for this Ligand-Target Pair
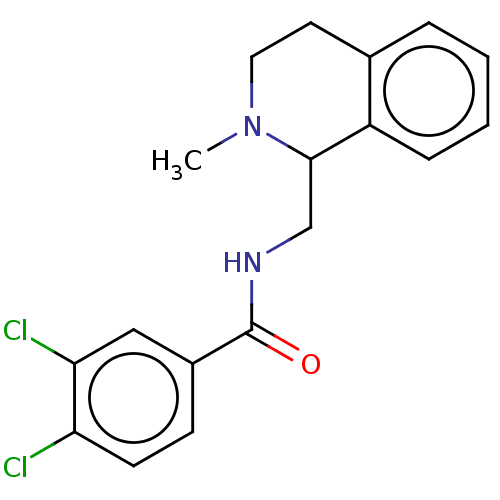
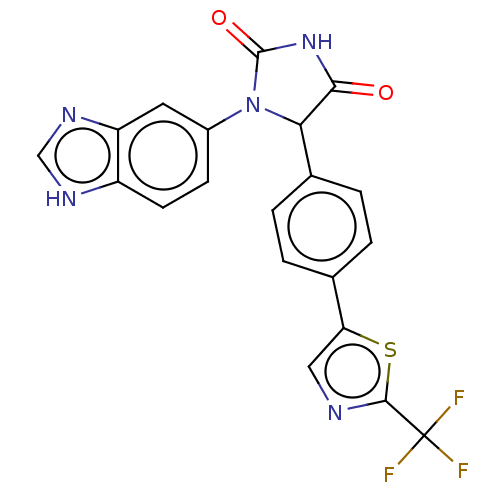
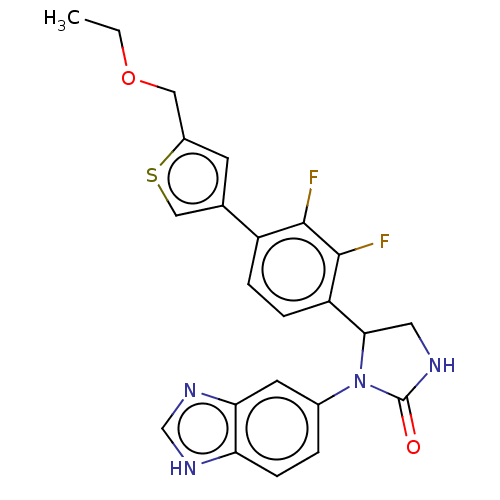
Affinity DataKi: 0.000120nMAssay Description:Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cellsMore data for this Ligand-Target Pair
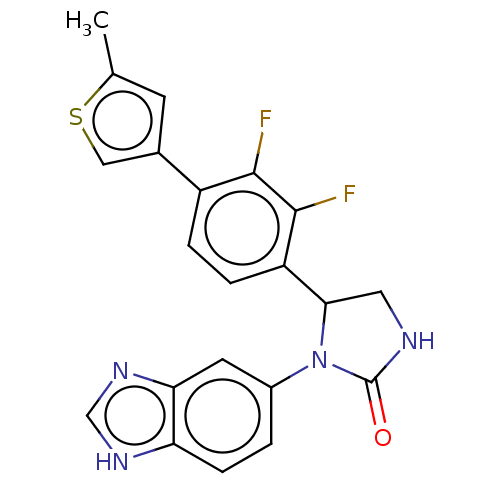
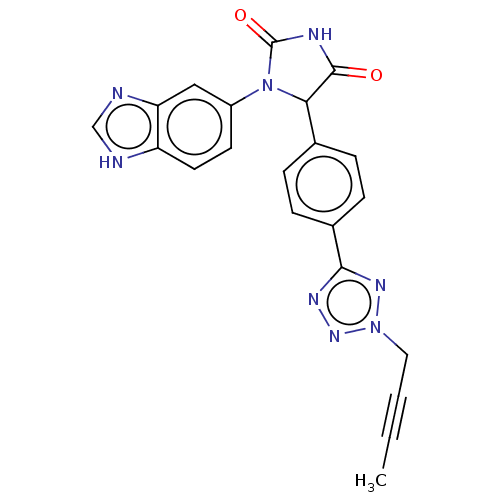
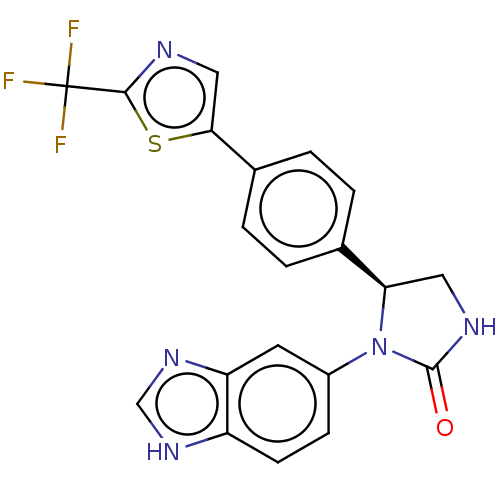
Affinity DataKi: 0.000630nMAssay Description:Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cellsMore data for this Ligand-Target Pair
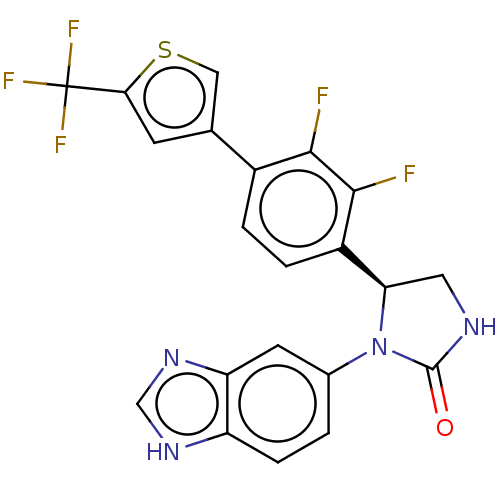
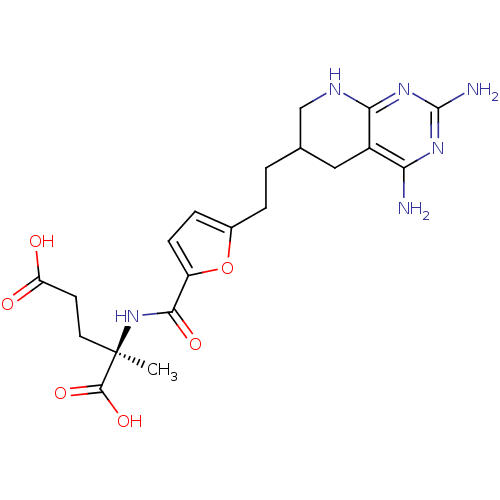
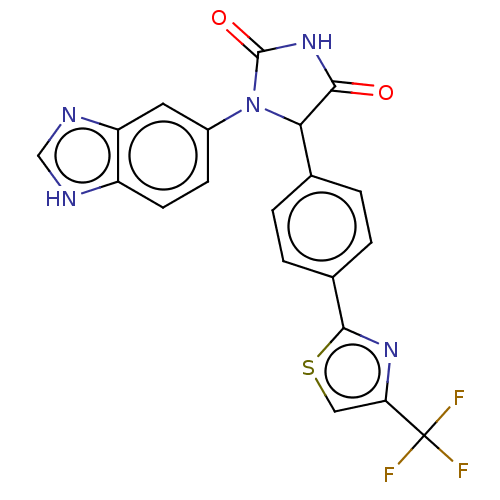
Affinity DataKi: 0.00500nMAssay Description:Binding affinity of the compound against Recombinant human dihydrofolate reductase (DHFR)More data for this Ligand-Target Pair
Affinity DataKi: 0.120nMAssay Description:Binding affinity of the compound against Recombinant human dihydrofolate reductase (DHFR)More data for this Ligand-Target Pair
Affinity DataKi: 0.290nMAssay Description:Inhibitory activity against recombinant human dihydrofolate reductase (DHFR)More data for this Ligand-Target Pair
Affinity DataKi: 0.690nMAssay Description:Inhibitory activity against recombinant human dihydrofolate reductase (DHFR)More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
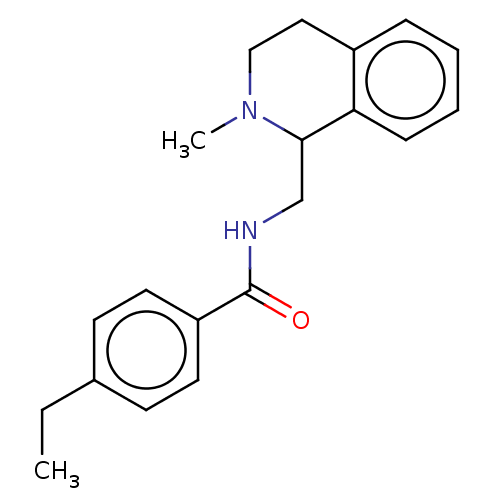
Affinity DataKi: 2nMAssay Description:Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 minsMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 minsMore data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
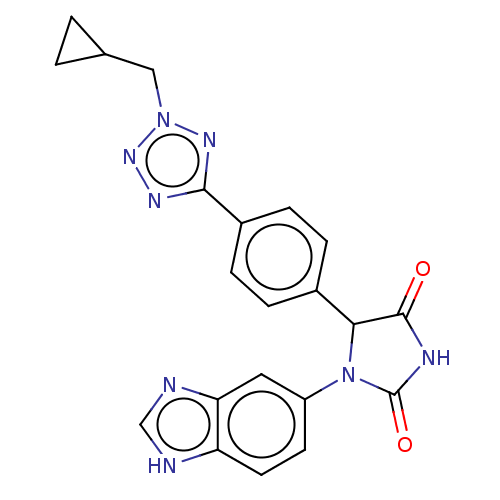
Affinity DataKi: 4nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
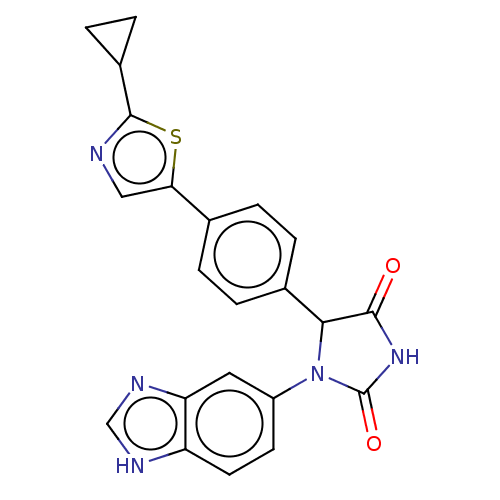
Affinity DataKi: 4nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
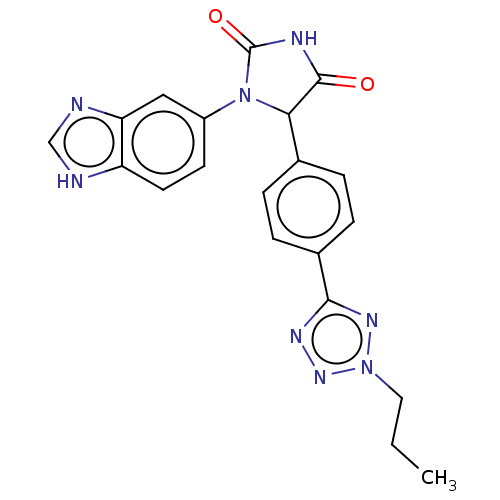
Affinity DataKi: 4nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: 4.70nMAssay Description:Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 5nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: 5.30nMAssay Description:Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 minsMore data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 6nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 6nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetRho-associated protein kinase 1(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Inhibition of human ROCK1 by homogenous luciferase assayMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: 6.5nMAssay Description:Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 minsMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: 6.90nMAssay Description:Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 minsMore data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 7nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 7nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 8nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 8nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 9nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
Affinity DataKi: 9.90nMAssay Description:Inhibitory activity against recombinant human dihydrofolate reductase (DHFR)More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 10nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 10nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 10nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 11nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 12nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 13nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 13nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 16nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 16nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 18nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 19nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 20nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 21nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 23nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 24nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibition of human placental adenosine kinaseMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Compound was evaluated for competitive inhibition of recombinant mouse thymidylate synthaseMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: 31nMAssay Description:Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 minsMore data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 32nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 36nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: 36nMAssay Description:Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 minsMore data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 39nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Inhibition of human placental adenosine kinaseMore data for this Ligand-Target Pair
TargetGlutaminyl-peptide cyclotransferase(Homo sapiens (Human))
National Health Research Institutes
US Patent
National Health Research Institutes
US Patent
Affinity DataKi: 49nMAssay Description:An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3...More data for this Ligand-Target Pair







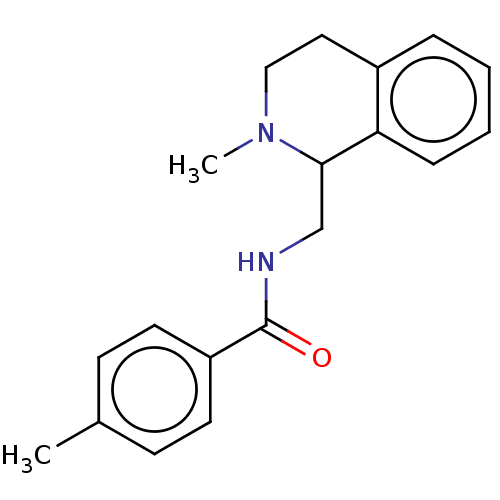

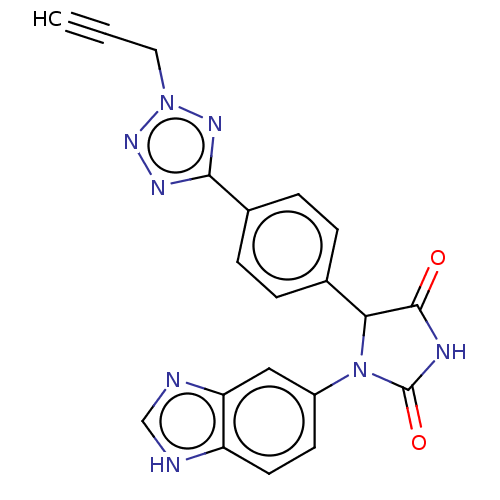
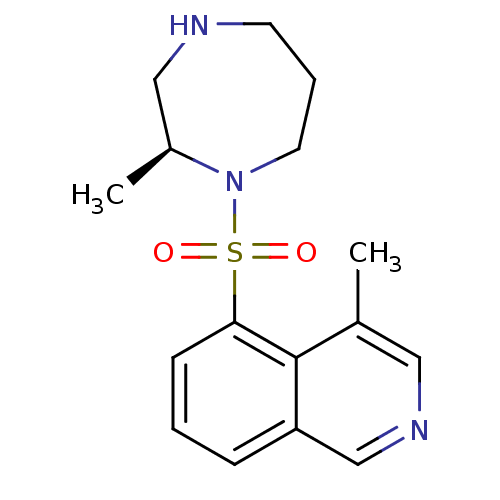
 3D Structure (crystal)
3D Structure (crystal)