Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Peroxisome proliferator-activated receptor alpha
Ligand
BDBM28700
Substrate
n/a
Meas. Tech.
ChEMBL_304204 (CHEMBL829744)
EC50
>10000±n/a nM
Citation
 Devasthale, PV; Chen, S; Jeon, Y; Qu, F; Shao, C; Wang, W; Zhang, H; Cap, M; Farrelly, D; Golla, R; Grover, G; Harrity, T; Ma, Z; Moore, L; Ren, J; Seethala, R; Cheng, L; Sleph, P; Sun, W; Tieman, A; Wetterau, JR; Doweyko, A; Chandrasena, G; Chang, SY; Humphreys, WG; Sasseville, VG; Biller, SA; Ryono, DE; Selan, F; Hariharan, N; Cheng, PT Design and synthesis of N-[(4-methoxyphenoxy)carbonyl]-N-[[4-[2-(5- methyl-2-phenyl-4-oxazolyl)ethoxy]phenyl]methyl]glycine [Muraglitazar/BMS-298585], a novel peroxisome proliferator-activated receptor alpha/gamma dual agonist with efficacious glucose and lipid-lowering activities. J Med Chem 48:2248-50 (2005) [PubMed] Article
Devasthale, PV; Chen, S; Jeon, Y; Qu, F; Shao, C; Wang, W; Zhang, H; Cap, M; Farrelly, D; Golla, R; Grover, G; Harrity, T; Ma, Z; Moore, L; Ren, J; Seethala, R; Cheng, L; Sleph, P; Sun, W; Tieman, A; Wetterau, JR; Doweyko, A; Chandrasena, G; Chang, SY; Humphreys, WG; Sasseville, VG; Biller, SA; Ryono, DE; Selan, F; Hariharan, N; Cheng, PT Design and synthesis of N-[(4-methoxyphenoxy)carbonyl]-N-[[4-[2-(5- methyl-2-phenyl-4-oxazolyl)ethoxy]phenyl]methyl]glycine [Muraglitazar/BMS-298585], a novel peroxisome proliferator-activated receptor alpha/gamma dual agonist with efficacious glucose and lipid-lowering activities. J Med Chem 48:2248-50 (2005) [PubMed] Article More Info.:
Target
Name:
Peroxisome proliferator-activated receptor alpha
Synonyms:
NR1C1 | Nuclear receptor subfamily 1 group C member 1 | PPAR | PPAR alpha/gamma | PPAR-alpha | PPARA | PPARA_HUMAN | Peroxisome Proliferator-Activated Receptor alpha | Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor alpha (PPAR alpha)
Type:
Enzyme
Mol. Mass.:
52222.08
Organism:
Homo sapiens (Human)
Description:
Q07869
Residue:
468
Sequence:
MVDTESPLCPLSPLEAGDLESPLSEEFLQEMGNIQEISQSIGEDSSGSFGFTEYQYLGSCPGSDGSVITDTLSPASSPSSVTYPVVPGSVDESPSGALNIECRICGDKASGYHYGVHACEGCKGFFRRTIRLKLVYDKCDRSCKIQKKNRNKCQYCRFHKCLSVGMSHNAIRFGRMPRSEKAKLKAEILTCEHDIEDSETADLKSLAKRIYEAYLKNFNMNKVKARVILSGKASNNPPFVIHDMETLCMAEKTLVAKLVANGIQNKEAEVRIFHCCQCTSVETVTELTEFAKAIPGFANLDLNDQVTLLKYGVYEAIFAMLSSVMNKDGMLVAYGNGFITREFLKSLRKPFCDIMEPKFDFAMKFNALELDDSDISLFVAAIICCGDRPGLLNVGHIEKMQEGIVHVLRLHLQSNHPDDIFLFPKLLQKMADLRQLVTEHAQLVQIIKKTESDAALHPLLQEIYRDMY
Inhibitor
Name:
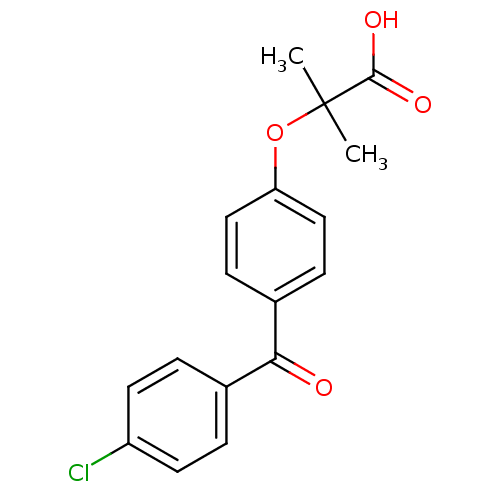
BDBM28700
Synonyms:
2-(4-(4-Chlorobenzoyl)phenoxy)-2-methylpropionic acid | 2-{4-[(4-chlorophenyl)carbonyl]phenoxy}-2-methylpropanoic acid | CHEMBL981 | FENOFIBRIC ACID | FIBRICOR | Fenofibrate | LF 153 | alpha-1081 | procetofenic acid
Type:
Small organic molecule
Emp. Form.:
C17H15ClO4
Mol. Mass.:
318.752
SMILES:
CC(C)(Oc1ccc(cc1)C(=O)c1ccc(Cl)cc1)C(O)=O